

A 57 year old male was seen at a local clinic with a year-long history of productive cough, occasionally with some blood spots. The patient had no fever or weight loss. He lived in Peru and had no history of exposure to tuberculosis. Routine AFB sputum examinations were negative; he was placed on therapy for TB. Approximately 2 months later, he had not improved. Subsequent sputum examinations revealed brown specks in the specimen and the images seen below. Structures measure approximately 90 by 52 microns.
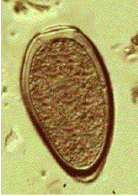
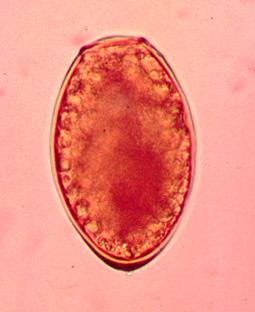
What infection most likely matches these images? Based on the history above why might there be some discussion regarding foodborne illness? Why do you think the AFB sputum examinations were negative?
SCROLL DOWN FOR A COMPLETE DISCUSSION OF THE CASE
Note the key characteristics: large helminth egg (operculated with opercular shoulders and thickened shell at the abopercuar end). Also, if Paragonimus infection is suspected, then potential routes of infected crab ingestion should be considered. In Peru, crab “ceviche” is the normal mode of transmission. Remember that the processing of sputum for AFB staining will routinely destroy these helminth eggs.
Paragonimus spp., also known as lung flukes, cause paragonimiasis in humans. The adults encapsulate in the lungs and are occasionally found in other body sites.
The infections caused by lung trematodes are foodborne and have considerable economic and public health impact. It is estimated that more than 50 million people have acquired foodborne trematode infections. Of great public health concern is the misdiagnosis of tuberculosis in those infected with Paragonimus spp.
Depending on the endemic area, a number of species are confirmed or thought to infect humans. These include Paragonimus bangkokensis, Paragonimus harinasutai, Paragonimus heterotremus, Paragonimus hueitungensis (may be included with P. skrjabini), Paragonimus kellicotti (North/South America), Paragonimus macrorchis, Paragonimus mexicanus (Central/South America), Paragonimus proliferus, Paragonimus pseudoheterotremus, Paragonimus siamensis, Paragonimus skrjabini, Paragonimus vietnamensis, and Paragonimus westermani. As molecular testing expands, the species designations may be changed.
The adult worm is a plump, ovoid, reddish brown fluke found encapsulated in the lung. Eggs deposited by the worms are ovoid, brownish yellow, unembryonated, and thick-shelled, with an operculum at one end and opercular shoulders. The eggs measure 80 to 120 µm by 45 to 65 µm. Paragonimus eggs are often confused with Diphyllobothrium eggs because they are operculated, unembryonated, and somewhat similar in size. However, unlike Diphyllobothrium eggs, Paragonimus eggs have opercular shoulders and a thickened shell at the abopercular end. Eggs escape from the encapsulated tissue through the bronchioles, are coughed up and voided in the sputum, or are swallowed and passed out in the feces. The eggs hatch in the water in 2 to 3 weeks, releasing a miracidium to infect a susceptible snail host. Cercariae are released after sporocyst and redia generations. Crabs and crayfish are infected by cercariae via the gill chamber or on ingestion of an infected snail. Cercariae encyst in the gill vessels and muscles. Humans are infected by ingesting uncooked crabs or crayfish containing metacercariae.
The metacercariae excyst in the duodenum and migrate through the intestinal wall into the abdominal cavity. The larvae migrate around or through the diaphragm into the pleural cavity and the lungs; they mature to adults in the vicinity of the bronchioles, where they discharge their eggs into the bronchial secretions, which can be swallowed (eggs appear in the stool). Although these worms are hermaphroditic, two worms are usually required for fertilization to occur. The worms can live as long at 20 years, but most die after about 6 years.
Migration of the larval forms through the intestinal wall into the abdominal cavity is generally not associated with any significant pathologic changes or symptoms. If the larvae remain in the abdomen, some patients may have abdominal pain, intra-abdominal masses, tenderness, fever, diarrhea, nausea, vomiting, and eosinophilia. Once the larvae have reached the peritoneal cavity, they begin to migrate through organs and tissues, producing localized hemorrhage and leukocytic infiltrates.
When the worms finally reach the lungs and mature, a pronounced tissue reaction occurs, with infiltration of eosinophils and neutrophils. A fibrotic capsule forms around the worm. The cysts contain purulent fluid with flecks or “iron filings” composed of brownish yellow eggs. Many of the cysts perforate into the bronchioles, releasing their contents of eggs, necrotic debris, metabolic by-products, and blood into the respiratory tract. The eggs may also enter the pulmonary tissue, or they may be carried by the circulatory system to other body sites, where they cause a granulomatous reaction.
Symptoms of paragonimiasis depend largely on the worm burden of the host and are usually insidious in onset and mild in patients with chronic infections. Light infections may be asymptomatic, although peripheral blood eosinophilia and lung lesions may be noted on X-ray examination. As the cysts rupture, a cough develops, with increased production of viscous blood-tinged sputum (rusty sputum with brown flecks, which may have a foul fish odor) and increasing chest pain. The patient may experience increasing dyspnea with chronic bronchitis and be misdiagnosed as having tuberculosis or bronchial asthma.
Individuals with symptoms of chronic cough, vague chest pains, and hemoptysis who have resided in an area where infections are endemic and have a history of eating raw crayfish or crabs should be suspected of having paragonimiasis. Paragonimus eggs can be detected in the sputum and the stool, and concomitant examinations should be performed to improve the overall detection rate. In many individuals in whom the infection is eventually confirmed, small numbers of eggs are present intermittently in the sputum and feces. For patients with light infections, up to seven sputum examinations have been recommended. Frequently, pulmonary paragonimiasis is misdiagnosed as pulmonary tuberculosis. The Ziehl-Neelsen method for detecting mycobacteria destroys Paragonimus eggs. It is important to remember that the typical findings of cough, hemoptysis, and eggs in the feces or sputum may be absent in patients with ectopic or pleural Paragonimus infection.
Paragonimus eggs may be confused with Diphyllobothrium latum eggs because of similarities in their size and shape. However, most Paragonimus eggs have opercular shoulders and a marked thickening at the abopercular end, unlike D. latum eggs.
The sedimentation concentration method should be used; because the eggs are operculated, they do not float in the zinc sulfate flotation concentration method. Remember that processing for AFB staining will destroy the helminth eggs.
It is important not to add too much iodine to the wet preparation, or the eggs will stain very darkly and resemble debris.
The wet preparation can be examined using the 10x (low-power) objective; these eggs are large enough that they can usually be seen under this magnification.
The wet preparation should not be so thick that the eggs are obscured by normal stool debris.
When looking at sputum specimens for eggs, it is necessary to carefully examine any blood-tinged flecks for eggs; the egg clusters have been described as looking like iron filings.
In light infections, multiple stool and sputum specimens may be needed before the eggs are detected.
If available, antigen or antibody detection may be helpful in confirming the infection. Use CT scans for brain type; biopsy for subcutaneous type.
There are approximately 200 million people at risk and 22 million people with paragonimiasis worldwide; a number of species are recognized as causing human disease. Reservoir hosts include dogs and cats in areas of endemic infection (the Far East and Africa). Eggs expectorated or passed in the feces in the vicinity of lakes or streams where the intermediate hosts live serve as the source of infection. Snail intermediate hosts include a number of different species. Humans contract the infection through ingestion of raw, undercooked, pickled, or wine-soaked crabs or crayfish; they may also become infected by the ingestion of uncooked meat from wild animals, such as wild boar. The migrating larvae in this meat may pass through the intestinal wall and continue their developmental cycle when eaten. Raw juice from crushed crayfish used as a home remedy for the treatment of measles has been a significant source of infection and may be a cause of cerebral paragonimiasis in children.
In most areas, the disease is primarily one of crab- or crayfish-eating mammals, with humans being incidental hosts. Measures devoted to public health education, warning people of the dangers of eating uncooked crabs or crayfish from areas of endemic infection, and sanitary care of utensils and fingers used to prepare food would reduce the risk of infection. Because the cycle depends on animals, the elimination of human paragonimiasis would have little effect on the overall prevalence of the disease.

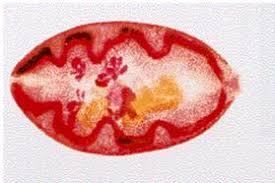
Crab containing infective forms. Adult Paragonimus fluke.
Garcia, L.S, 2016. Diagnostic Medical Parasitology, 6th Ed., ASM Press, Washington, D.C.
Keiser, J., and J. Utzinger. 2005. Emerging foodborne trematodiasis. Emerg. Infect. Dis. 11:1507–1514.
Mortality and Morbidity Weekly Report. 2010. Human paragonimiasis after eating raw or undercooked crayfish –Missouri, July 2006-September 2010. MMWR 59:1573-1576.
Procop, GW. 2009. North American paragonimiasis (caused by Paragonimus kellicotti) in the context of global paragonimiasis. Clin Microbiol Rev 22:415-446.
Each Quiz has a two section format: the first section will present the Quiz topic and the second section will provide a discussion of the answer and/or various options in response to the Quiz situation presented to the user. In some situations, there may be more than one correct response.
The content within this site is made possible through the extensive contribution of Lynne S. Garcia, M.S., MT(ASCP), CLS(NCA), BLM(AAB), F(AAM), Director, Consultantation and Training Services (Diagnostic Medical Parasitology and Health Care Administration). For additional information, she can be contacted at LynneGarcia2@verizon.net.
Reference: Garcia, L.S. 2015. Diagnostic Medical Parasitology, 6th Ed., ASM Press, Washington, D.C.