

A 45-year-old engineer presented to his physician with jaundice. He reported that a few days before he had developed a fever and also had diarrhea, nausea, and vomiting. He also indicated he had a cough about a week earlier. A few days later, he experienced right upper quadrant pain. On the day he presented to the physician, he had noticed his eyes were somewhat yellow. The patient works for an American-based engineering firm that has multiple contracts throughout South America; their main responsibilities include drilling for water and construction of irrigation canals in recently cleared land areas of several South American countries, including Bolivia, Argentina, Chile, and Peru. Periodically he spends time in these areas, usually eating whatever is available. Often the engineering job is adjacent to sheep and/or cattle raising regions.
On presentation, the patient appeared in no particular distress; however, he was jaundiced. The lungs and heart were normal. There was guarding and tenderness in the right upper quadrant. The liver was enlarged and tended down to 3 cm below the costal margin, with a span of 15 cm. There was no peripheral edema.
The white blood cell count was 18,500, with 35 PMNs, 10 lymphocytes, 3 monocytes, and 52 eosinophils. Stool examinations for parasites revealed the following:
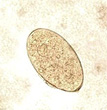
This object measured about 140 by 70 microns and was easily seen in the saline wet preparation examination.
Scroll Down for Answer and Discussion
Answer and Discussion of Quiz #19
The image presented in Diagnostic Quiz #19 is the following:
Comment: The patient's symptoms will reflect the phase of the infection, as well as the number of parasites present in the host. In the acute phase, symptoms may be present over a period of weeks to months. Metacercarial larvae do not produce significant pathologic damage until they begin to migrate through the liver parenchyma. The amount of damage depends on the worm burden of the host. Linear lesions of 1 cm or greater can be found. Hyperplasia of the bile ducts occurs, possibly as a result of toxins produced by the larvae. Symptoms associated with this migratory phase can include fever, epigastric and right upper quadrant pain, and urticaria. Asymptomatic infections appear to be more common in Peru.
In the more chronic phases of the disease, the patient generally has few to no symptoms once the flukes have lodged in the biliary passages. However, there may be some epigastric and right upper quadrant pain, diarrhea, nausea, vomiting, hepatomegaly, and jaundice. If the flukes are found in the extrahepatic biliary ducts, symptoms may mimic those seen in cholelithiasis. In the chronic phase, there tends to be some liver function abnormalities, as well as eosinophilia.
Once the worms have established themselves in the bile ducts and matured, they cause considerable damage from mechanical obstruction and metabolic by-products as well as obstruction. The degree of pathologic change depends on the number of flukes penetrating the liver. The infection produces hyperplasia of the biliary epithelium and fibrosis of the ducts with portal or total biliary obstruction. The gallbladder undergoes similar pathologic changes and may even harbor adult worms.
Symptoms and signs of infection during the late stages after egg production has begun are those associated with biliary obstruction and cholangitis. Acute epigastric pain, fever, pruritus, jaundice, hepatomegaly, and eosinophilia are common.
Life Cycle: Adult worms, which may live for 9 years in the bile ducts, produce eggs that are carried by the bile fluid into the intestinal lumen and passed into the environment with the feces. The eggs are unembryonated, operculated, large, ovoid, and brownish yellow. The miracidium develops within 1 to 2 weeks and escapes from the egg to infect the snail intermediate host, Lymnaea sp. Cercariae are liberated from the snail after the production of a sporocyst generation and two or three rediae generations. Cercariae encyst on water vegetation, e.g., watercress. Humans are infected by ingestion of uncooked aquatic vegetation on which metacercariae are encysted. Metacercariae excyst in the duodenum and migrate through the intestinal wall into the peritoneal cavity. The larvae enter the liver by penetrating the capsule (Glisson's capsule) and wander through the liver parenchyma for up to 9 weeks. The larvae finally enter the bile ducts, where they mature and produce eggs, which are passed out in feces. The adult worms can attain a length of >1 in. (1 in. = 2.54 cm) and a width of about 0.5 in.
Epidemiology and Control: Fascioliasis is a cosmopolitan disease found where there is close association of livestock, humans and snails. The largest number of infections has been reported from Bolivia, Ecuador, Egypt, France, Iran, Peru and Portugal. Although animal fascioliasis is endemic throughout the Americas, human infections are rare in most countries. Reservoir hosts include herbivores such as cattle, goats and sheep. Animal fascioliasis is a major veterinary problem in Europe. The infection is contracted by the ingestion of metacercariae encysted on uncooked water plants, such as watercress. Watercress is the main source of infection, and in many countries wild watercress is touted as a healthy natural food. In many areas of the world, animal manure is used as the primary fertilizer for cultivation of watercress.
Prevention may be accomplished by public health education in areas where infections are endemic, stressing the dangers of eating watercress grown in the wild where animals and snails are abundant. Other measures have included mollusciciding, treatment of infected animals, and draining of pasture lands.
Fasciola hepatica, Key Points - Laboratory Diagnosis
Treatment: Although praziquantel is sometimes effective at a dose of 25 mg/kg taken after each meal for 2 days, it does not appear to be effective in treating cases of infection in Egypt. Treatment with 30 to 50 mg/kg of biothionol on alternate days for 10 to 15 doses is recommended. Triclabendazole at 10 mg/kg as a single dose is also recommended, but may not be as easily obtained. The drug is given orally in single or multiple doses and has few side effects. The drug acts by inhibiting protein synthesis in F. hepatica and will probably become the drug of choice.
|
|
|
Three images of Fasciola hepatica eggs from a routine stool examination (wet preparation). The first image is the egg seen above in a saline wet preparation (note the presence of the operculum at the top of the egg). The middle image shows an egg photographed in a saline wet mount, but at a somewhat higher magnification (note the open operculum). The image on the far right shows an egg photographed from an iodine wet mount; the operculum is not clearly visible
|
|
The image on the left shows an adult worm within the bile duct (curved "U" shape); the image on the right shows the adult fluke within the liver. The image on the right is shown at a larger magnification.
References:
Each Quiz has a two section format: the first section will present the Quiz topic and the second section will provide a discussion of the answer and/or various options in response to the Quiz situation presented to the user. In some situations, there may be more than one correct response.
The content within this site is made possible through the extensive contribution of Lynne S. Garcia, M.S., MT(ASCP), CLS(NCA), BLM(AAB), F(AAM), Director, Consultantation and Training Services (Diagnostic Medical Parasitology and Health Care Administration). For additional information, she can be contacted at LynneGarcia2@verizon.net.
Reference: Garcia, L.S. 2015. Diagnostic Medical Parasitology, 6th Ed., ASM Press, Washington, D.C.