

A 9-year-old male was seen with complaints of watery diarrhea, abdominal cramping, and a low-grade fever. His symptoms began a few days after the family vacation to various ball parks and zoos. Routine ova and parasite examinations were ordered. The examination included a wet mount of the concentration sediment and microscopic examination of a Trichrome-stained fecal smear. The following images were seen. The image on the left was seen in a wet mount; the image has been expanded to show morphological details. Note: Wet mounts are normally examined using the low power (10x) and high dry power (40x) objectives; oil immersion is not recommended. This image would appear much smaller microscopically; the image has been enlarged. The image on the right is from the Trichrome-stained smear. These structures were seen using the oil immersion objective (100x).
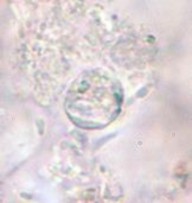
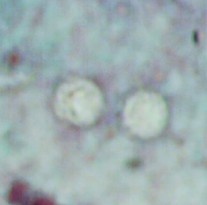
What parasitic infection might be involved?
Scroll Down for Answer and Discussion
The images presented in Diagnostic Quiz #88 are the following:
The objects shown in this case were Cryptosporidium spp. oocysts. Oocysts in wet mounts may not be seen at all unless the number of oocysts is very high. In a Trichrome stained smear, often the oocysts appear as “ghost cells”
that do not stain well. Again, unless the number of oocysts is high, the organisms may not be recognized as possible parasites.
Cryptosporidiosis is well recognized as a disease in humans, particularly in those who are in some way immunosuppressed or immunodeficient.
Cryptosporidium has been implicated as one of the more important opportunistic agents seen in patients with AIDS. Also, reports of respiratory tract and biliary tree infections confirm that the developmental stages of this organism are not always confined to the gastrointestinal tract. Oocysts recovered in clinical specimens are difficult to see without special staining techniques, such as the modified acid-fast, Kinyoun’s, and Giemsa methods, or the newer direct FA assay or EIA methods. The four sporozoites may be seen within the oocyst wall in some of the organisms, although in freshly passed specimens they are not always visible. The standard O&P concentration sedimentation is recommended (500 × g for 10 min), with the concentrate sediment being used for all smears and for the FA procedure.
Shorter centrifugation at lower speeds (often mentioned in some procedures) may not guarantee recovery of the oocysts. Multiple stool specimens may have to be examined to diagnose the infection, particularly when
formed stool specimens are being tested. (See images below)
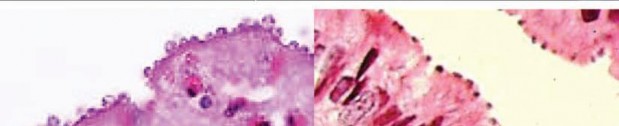
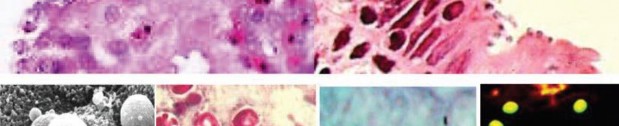
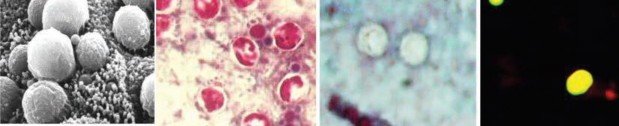
Cryptosporidium spp. (Top row) Artist’s rendering of Cryptosporidium oocysts containing sporozoites; sporozoites being released from the oocyst (courtesy of CDC PHIL [https://www.cdc.gov/parasites/crypto/] and CDC Newsroom [https://www.cdc.gov/media/releases/2017/p0518-cryptosporidium-outbreaks. html]). (Middle row) Cryptosporidium sp. oocysts along the microvillous surface; developing Cryptosporidium at the microvillous surface (courtesy of Armed Forces Institute of Pathology). (Bottom row) Scanning electron micrograph of Cryptosporidium oocysts (4 to 6 μm); oocysts stained with modified acid-fast stain (these oocysts stain more consistently than those of Cyclospora cayetanensis; in some oocysts, the sporozoites can be
seen; oocysts are infectious even if the sporozoites are not visible); two “ghost” cells (routine Wheatley’s trichrome stain; oocysts do not stain well); results of an FA assay—the large object is a Giardia lamblia cyst, and
the smaller objects are Cryptosporidium sp. oocysts.

Garcia, LS, 2016. Diagnostic Medical Parasitology, 6th Ed., ASM Press, Washington, DC.
Gibson AR, Striepen B. 2018. Cryptosporidium. Curr Biol Mar 5;28(5):R193-R194. doi: 10.1016/j.cub.2017.11.070
Widmer G, Carmena D, Kvac M et al. 2020. Update on Cryptosporidium spp.: highlights from the Seventh International Giardia and Cryptosporidium Conference. Parasite. 2020;27:14. doi: 10.1051/parasite/2020011. Epub 2020 Mar 13.
Each Quiz has a two section format: the first section will present the Quiz topic and the second section will provide a discussion of the answer and/or various options in response to the Quiz situation presented to the user. In some situations, there may be more than one correct response.
The content within this site is made possible through the extensive contribution of Lynne S. Garcia, M.S., MT(ASCP), CLS(NCA), BLM(AAB), F(AAM), Director, Consultantation and Training Services (Diagnostic Medical Parasitology and Health Care Administration). For additional information, she can be contacted at LynneGarcia2@verizon.net.
Reference: Garcia, L.S. 2015. Diagnostic Medical Parasitology, 6th Ed., ASM Press, Washington, D.C.