

A 34 year old female was admitted to the hospital with complaints of fever, fatigue, generalized myalgia, abdominal pain, and a slightly elevated eosinophilia. This patient had lived in Korea and recently come to the United States to visit relatives. She had no significant history of medical problems, other than a lingering cough and occasional blood-tinged sputum. She received anti-tuberculosis therapy. Two months later she was seen again.
Radiography results revealed multiple mass-like opacities in the right lung. The patient was suspected of having a metastatic lung tumor. However, the results of an ELISA test was strongly suggestive of a parasitic infection.
Bronchoscopic and cytological examination of the sputum were not significant.
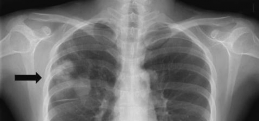

What parasitic infection might be involved? Based on the suspected diagnosis, why were no eggs found in the sputum?
Scroll Down for Answer and Discussion
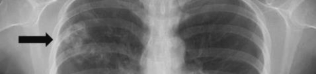
The image presented in Diagnostic Quiz #86 is the following:
Since the x-ray was not definitive, appropriate therapy with praziquantel was administered based on the result of the detection of antibodies in the patient’s serum to Paragonimus westermani (lung fluke). The followup chest X-ray after 4 months showed a decrease in the size of multiple nodules, and at 6 months, the nodules were markedly
diminished. It is not unusual for eggs to be absent from pulmonary and/or stool specimens.

Comments on the Patient: Although this case represents the possibility of several conditions, in other patients the diagnosis can be confirmed by the presence of typical Paragonimus in stool, sputum, or BALs (see below). Note the thickened abopercular end (arrow) and operculum (oval).


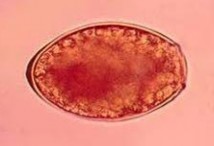
Comments on Paragonimus westermani: The adult worm is a plump, ovoid, reddish brown fluke found encapsulated in the lung. Eggs deposited by the worms are ovoid, brownish yellow, unembryonated, and thick-shelled, with an operculum at one end and opercular shoulders. The eggs measure 80 to 120 µm by 45 to 65 µm. P. westermani eggs are often confused with Diphyllobothrium eggs because they are operculated, unembryonated, and somewhat similar in size. However, unlike Diphyllobothrium eggs, P. westermani eggs have opercular shoulders and a thickened shell at the abopercular end (See above). Eggs escape from the encapsulated tissue
through the bronchioles, are coughed up and voided in the sputum, or are swallowed and passed out in the feces. The eggs hatch in the water in 2 to 3 weeks, releasing a miracidium to infect a susceptible snail host. Cercariae are released after sporocyst and redia generations. Crabs and crayfish are infected by cercariae via the gill chamber or on ingestion of an infected snail. Cercariae encyst in the gill vessels and muscles. Humans are infected by ingesting uncooked crabs or crayfish containing metacercariae. The metacercariae excyst in the duodenum and migrate through the intestinal wall into the abdominal cavity. The larvae migrate around or through the diaphragm into the pleural cavity and the lungs; they mature to adults in the vicinity of the bronchioles, where they discharge their eggs into the bronchial secretions. Although these worms are hermaphroditic, two worms are usually required for fertilization to occur. The worms can live as long at 20 years, but most die after about 6 years.
Although we generally think of infected raw crabs or crayfish as the most common sources of infection, a number of other foods and cultural practices can be implicated as well, including the ingestion of metacercariae in the flesh of paratenic hosts.
Symptoms of paragonimiasis depend largely on the worm burden of the host and are usually insidious in onset and mild in patients with chronic infections. Light infections may be asymptomatic, although peripheral blood eosinophilia and lung lesions may be noted on X-ray examination. As the cysts rupture, a
cough develops, with increased production of viscous blood-tinged sputum (rusty sputum, which may have a foul fish odor) and increasing chest pain. The patient may experience increasing dyspnea with chronic bronchitis and be misdiagnosed as having tuberculosis or bronchial asthma). The individual generally has a moderately high peripheral blood eosinophilia and leukocytosis, with elevated levels of IgG and IgE in serum. Although some patients exhibit symptoms continuously, others may remain asymptomatic for weeks to months between periods of hemoptysis.
P. mexicanus, and occasionally P. westermani. Cysts have been detected in the liver, intestinal wall, muscles, peritoneum, and brain. The most serious consequences of paragonimiasis are the cerebral complications, which are commonly found in younger age groups. Unlike adult flukes in other extrapulmonary sites, worms found in the brain usually contain eggs. The worms probably migrate from ruptured lung cysts and travel through the soft tissues surrounding veins into the brain area. The worms eventually encapsulate, but before being walled off they cause necrosis within the brain tissue, as well as possible cerebral hemorrhage, edema, and meningitis.
Most patients with cerebral or other extrapulmonary lesions have an associated lung lesion or a history of lung disease. Symptoms include fever, headache, nausea, vomiting, visual disturbances, motor weakness, localized or generalized paralysis, and possibly death. More than half of these patients also exhibit personality changes, possible disorientation, and a general decline in cognitive function. Depending on the central nervous system location, symptoms may also include paraplegia, sensory loss, or vision problems. Specific sites can include the cerebral cortex, cerebellum, basal ganglia, medulla oblongata, and spinal cord. When a worm dies, the lesion cavity becomes filled with necrotic material. Cerebral paragonimiasis can be difficult to differentiate from brain disease caused by other parasites such as Schistosoma japonicum, Gnathostoma spinigerum, or Angiostrongylus cantonensis, as well as other infectious agents such as bacteria or viruses.
Laboratory Tests. Individuals with symptoms of chronic cough, vague chest pains, and hemoptysis who have resided in an area where infections are endemic and have a history of eating raw crayfish or crabs should be suspected of having paragonimiasis. Paragonimus eggs can be detected in the sputum and the stool, and both specimens should be examined to improve
the overall detection rate. In many individuals in whom the infection is eventually confirmed, small numbers of eggs are present intermittently in the sputum and feces. For patients with light infections, up to seven sputum examinations have been recommended. Frequently, pulmonary paragonimiasis is misdiagnosed as pulmonary tuberculosis. The Ziehl-Neelsen method for detecting mycobacteria destroys Paragonimus eggs. It is important to remember that the typical findings of cough, hemoptysis, and eggs in the feces or sputum may be absent in patients with ectopic or pleural Paragonimus infection.
Immunodiagnostic Tests. The various immunological tests which have been evaluated are intradermal (ID) test, complement fixation test (CFT), immunodiffusion, indirect hemagglutination test (IHA), enzyme-linked immunosorbent assay (ELISA), dot-ELISA, and Western blot. Complement fixation has been used to diagnose active infections; however, the test becomes negative soon after the death of the worms. An immunoblot assay for the detection of antibodies to P. westermani by using adult worm homogenates is highly sensitive and specific. ELISA with adult excretory-secretory antigens is very sensitive for detecting parasite-specific IgG and IgE. Pleural effusion fluid is more suitable than serum for detection of infections. The ELISA tests are most widely used for serological diagnosis of paragonimiasis due to their high sensitivity and specificity. However, ELISA tests are more expensive, time-consuming and require costly equipment and experienced persons; frequently, the reagents and antigens are not commercially available. Dot ELISA has also been used to detect parasite-
specific antigen in human sera. Monoclonal antibodies used to detect species-specific and stage-specific antigens are highly sensitive and specific for the detection of active infections). The serologic assays are available in areas of endemicity or in specialized diagnostic centers. Most of these assays involve nonstandardized reagents and have not been used in clinical trials. An intradermal test is also available for epidemiologic surveys in areas of endemicity; however, positive reactions persist for years after parasitologic cures.
Rapid Test. A dot-immunogold filtration assay (DIGFA) kit was developed in China for anti-P. westermani antibody detection. This test is simple and rapid and does not require any special devices and/or experienced technicians; results are obtained within 10 min. This kit was prepared using P. westermani antigen and reported in China to have the sensitivity and specificity up to 99 and 92 per cent, respectively.
Paragonimus eggs may be confused with Diphyllobothrium latum eggs because of similarities in their size and shape. However, most Paragonimus eggs have opercular shoulders and a marked thickening at the abopercular end, unlike D. latum eggs.
The sedimentation concentration method should be used; because the eggs are operculated, they do not float in the zinc sulfate flotation concentration method.
It is important not to add too much iodine to the wet preparation, or the eggs will stain very darkly and resemble debris.
The wet preparation can be examined using the 10x (low-power) objective; these eggs are large enough that they can be seen using 10x.
The wet preparation should not be so thick that the eggs are obscured by normal stool debris.
When looking at sputum specimens for eggs, it is necessary to carefully examine any blood-tinged flecks for eggs; the egg clusters have been described as looking like iron filings.
In light infections, multiple stool and sputum specimens may be needed before the eggs are detected.
If available, antigen or antibody detection may be helpful in confirming the infection.
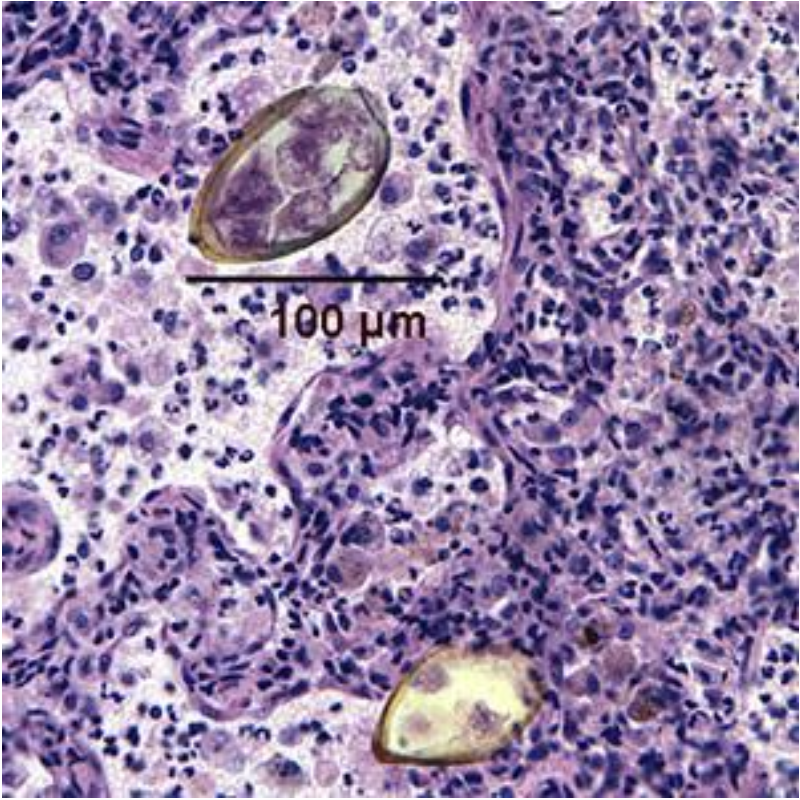
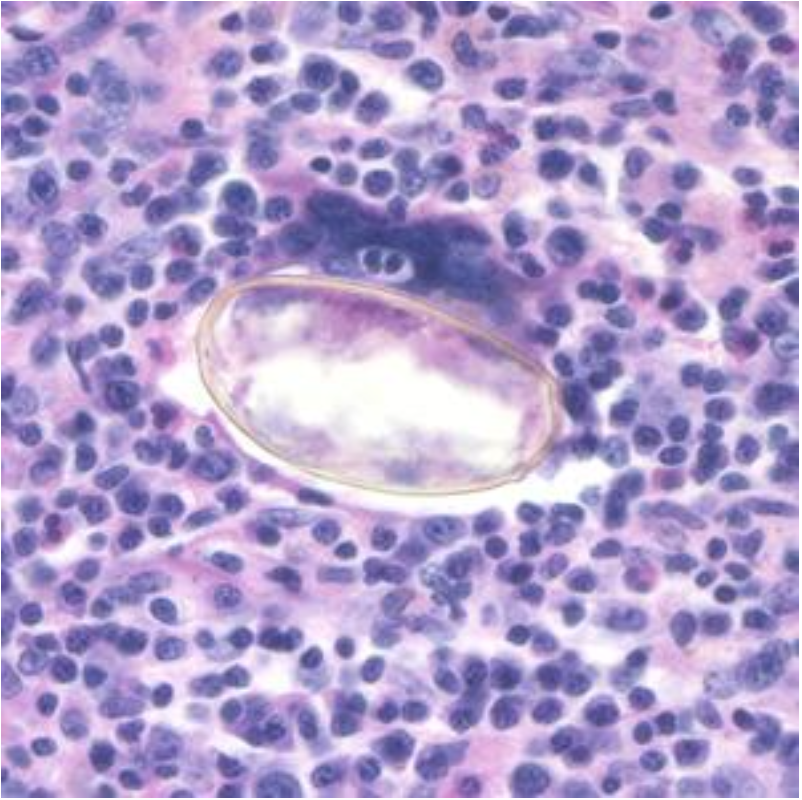
Eggs from lung biopsy (H&E); 80-90 by 40-45 microns.
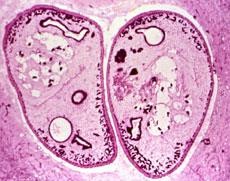
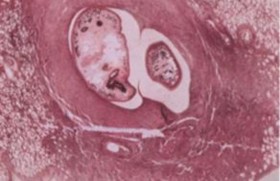
Adults found in pairs in lung cysts; one of the intermediate hosts is a crayfish.
Garcia, LS, 2016. Diagnostic Medical Parasitology, 6th Ed., ASM Press, Washington, DC.
Blair, D., Z. Chang, M. Chen, A. Cui, B. Wu, T. Agatsuma, M. Iwagami,
D. Corlis, C. Fu, and X. Zhan. 2005. Paragonimus skrjabini Chen, 1959 (Digenea: Paragonimidae) and related species in eastern Asia: a
combined molecular and morphological approach to identification and taxonomy. Syst. Parasitol. 60:1–21.
Toscano, C., Y. S. Hai, P. Nunn, and K. E. Mott. 1995. Paragonimiasis and tuberculosis, diagnostic confusion: a review of the literature. Trop. Dis. Bull. 92:R1–R26.
Brown, W. J., and M. Voge. 1982. Neuropathology of Parasitic Infections. Oxford University Press, Oxford, United Kingdom.
Slemenda, S. B., S. E. Maddison, E. C. Jong, and D. D. Moore. 1988. Diagnosis of paragonimiasis by immunoblot. Am. J. Trop. Med. Hyg. 39:469–471.
Ikeda, T., Y. Oikawa, M. Owhashi, and Y. Nawa. 1992. Parasite-specific IgE and IgG levels in the serum and pleural effusion of Paragonimiasis westermani patients. Am. J. Trop. Med. Hyg. 47:104–107.
Zihao, Z., S. Yiping, and W. F. Piessens. 1991. Characterization of stage-specific antigens of Paragonimus westermani. Am. J. Trop. Med. Hyg. 44:108–115.
Zhang, Z., Y. Zhang, Z. Shi, K. Sheng, L. Lui, Z. Hu, and W. F. Piessens. 1993. Diagnosis of active Paragonimus westermani infections with a monoclonal antibody-based antigen detection assay. Am. J. Trop. Med. Hyg. 49:329–334.
Singh, TS, H Sugiyama, A Rangsiruji. 2012. Paragonimus & paragonimiasis in India. Indian J Med Res 136:192-204.
Each Quiz has a two section format: the first section will present the Quiz topic and the second section will provide a discussion of the answer and/or various options in response to the Quiz situation presented to the user. In some situations, there may be more than one correct response.
The content within this site is made possible through the extensive contribution of Lynne S. Garcia, M.S., MT(ASCP), CLS(NCA), BLM(AAB), F(AAM), Director, Consultantation and Training Services (Diagnostic Medical Parasitology and Health Care Administration). For additional information, she can be contacted at LynneGarcia2@verizon.net.
Reference: Garcia, L.S. 2015. Diagnostic Medical Parasitology, 6th Ed., ASM Press, Washington, D.C.