

ANSWER: The formalin-ethyl acetate concentration procedure cannot be used to demonstrate any type of motility since the organisms have already been preserved in formalin prior to being concentrated. This procedure is designed to recover helminth eggs and protozoan cysts. Accurate identification of many of the protozoan cysts should be confirmed using a permanent stained smear that can be examined using the oil immersion lens for a total magnification of 1,000X. The correct answer is B.
ANSWER: The Baermann concentration procedure is designed to recover Strongyloides stercoralis larvae from fecal specimens. Rarely would one be able to recover Trichinella larvae from the stool. Also, Ascaris lumbricoides eggs are passed in the stool in the unembryonated stage. Enterobius vermicularis eggs are deposited on the perianal skin when the female worm migrates from the anus to oviposit. Consequently, these eggs are normally recovered from the perianal areas, not in the stool. The correct answer is C.
ANSWER: The miracidial hatching test is used to demonstrate viability of Schistosoma eggs. The other eggs mentioned do not hatch when placed in dechlorinated water. The release of actively swimming larvae indicates egg viability. The correct answer is B.
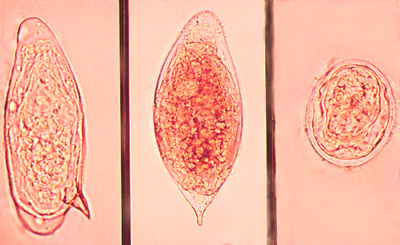
Left, S. mansoni; Middle, S. haematobium; Right, S. japonicum
ANSWER: The Harada-Mori and Petri dish culture techniques are used to recover the larvae of Strongyloides stercoralis. The correct answer is C.
ANSWER: The gravid proglottids of Taenia solium contain seven to thirteen uterine branches on each side of the main uterine stem. Taenia saginata usually has more than thirteen uterine branches on each side. This difference allows us to identify the Taenia spp. to the species level. The correct answer is B.
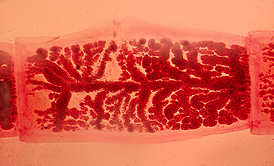
Taenia solium gravid proglottid; note number of uterine branches on each side.
ANSWER: Of those procedures listed above, the most obvious, and certainly the most life-threatening, would be the blood films for malaria. If the patient is suspected of having malaria, it is mandatory that the laboratory be equipped to diagnose and identify the organisms to the species level. Infections with Plasmodium falciparum can be fatal if not treated properly or daignosed early enough. Often, the patient may present with symptoms that may mimic many other disease states. Also, the number of organisms in the blood may not reflect the severity of the problem; i.e., a patient who is very ill may reveal very few orgnaisms on the peripheral blood film. The correct answer is D.
ANSWER: Although amebae may occasionally be recovered in the sputum, the trophozoites would not survive in a concentrated specimen. Therefore, the most appropriate answer would be paragonimiasis, since Paragonimus spp. live within the lungs and the eggs may be found in either the sputum or may be swallowed and recovered in the feces. A concentrated sputum specimen would not allow either the recovery of Trichinella spiralis larvae or microfilariae.The correct answer is A.
ANSWER: A 24-hour unpreserved urine specimen, properly concentrated, may be found to contain Schistosoma haematobium eggs. This is the most appropriate answer because the adult worms reside in the vesicle, prostatic, and uterine veins. The eggs are deposited in the walls of the bladder, for the most part, and usually the eggs will then break through into the lumen of the bladder and escape with the urine. The other three species listed as possible answers are not appropriate since these eggs are passed in the fecal material. Very rarely, Schistosoma haematobium worms may get into other veins and the eggs may accidentally be passed out in the feces. For the most part, however, they will be recovered in the 24-hour urine specimen. These specimens can then be examined either by centrifugation or by a hatching procedure in order to determine egg viability. The correct answer is B.
ANSWER: One of the most useful concentration techniques to demonstrate microfilariae is the membrane filter method. Baermann's concentration method is primarily designed to recover Strongyloides larvae, while the formalin-ethyl acetate concentration method would not be applicable for microfilariae since they are found in the peripheral blood. Also, the miracidial hatching method is designed primarily to demonstrate egg viability in schistosomiasis.The correct answer is C.
ANSWER: Examination of stool specimens would not be appropriate for Trichinella spiralis. After ingestion of infected meat, it might be possible to recover Trichinella larvae in the stool, but trichinosis is usually diagnosed on clinical grounds, history of raw pork or bear meat ingestion, and serological procedures. Eggs of Trichuris trichiura and Ascaris lumbricoides are recovered in the stool; Strongyloides stercoralis larvae could also be recovered in the stool. The correct answer is D.
ANSWER: Although Dientamoeba fragilis has no known cyst stage,now has a confirmed cyst stage, only about 1% of the stages on a patient smear are cysts and they are very difficult to identify. The organism is rarely seen on examination of concentrated material. The correct answer is B.
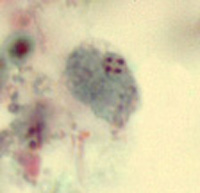
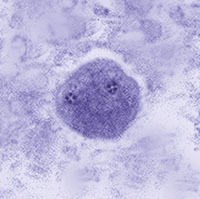
Dientamoeba fragilis trophozoites: Left, single nucleus; right, two nuclei
ANSWER: The direct saline mount is designed to demonstrate motility of protozoan trophozoites. The typical motility of Trichomonas vaginalis would be visible in a urine sediment, but this organism is not normally found in the stool. The correct answer is D.
ANSWER: The unfertilized eggs of Ascaris lumbricoides are usually very dense and will not float using the zinc sulfate procedure. The other organisms mentioned should be recovered using the zinc sulfate concentration with no difficulty. The correct answer is D.
ANSWER: Giemsa staining of thick and thin blood films requires prior fixation of the thin film. On a thin film, one would like to preserve the red cell morphology; and since Giemsa stain is an aqueous-based stain, these smears must be fixed in absolute methanol prior to staining. The correct answer is A.
ANSWER: The permanent stained smear (trichrome stain) for fecal specimens is designed to demonstrate protozoan cysts and trophozoites. Generally helminth eggs and larvae do not stain well and may resemble debris, while microsporidia and the coccidia require special stains, modified trichrome and modified acid-fast stains, respectively. The correct answer is B.
ANSWER: Although permanent stains of the organisms in lens care solutions and/or corneal biopsies are often successful in demonstrating the organisms, with a low parasitemia, the infection may not be diagnosed. The culture using non-nutrient agar plated seeded with a lawn of Escherichia coli on which the Acanthamoeba feed has proven to be the most sensitive method for confirming Acanthamoeba keratitis. The correct answer is C.
ANSWER: The SAF collection system gives the user the capability to perform the fecal concentration, the permanent stained smear, and some of the fecal immunoassays for Giardia lamblia and Cryptosporidium parvum. The correct answer is C.
ANSWER: If the regular decolorizer (acid alcohol) used for AFB work is used when staining for coccidia, too much stain is removed and the oocysts may not be visible. This is particularly true when staining Cyclospora cayetanensis. Consequently, a weaker decolorizer solution is recommended. The stain type and concentration and staining times are similar for both AFB and oocysts. The correct answer is B.
ANSWER: Of the parasites listed above, only Strongyloides stercoralis and Giardia lamblia both reside in the duodenum. Both of these organisms may be difficult to recover with the routine fecal examination; however, they may be recovered by sampling duodenal contents. The correct answer is D.
ANSWER: Modified trichrome stains are used primarily to demonstrate microsporidian spores. They are very refractive to staining, so the modified trichrome stain contains 10 times the amount of the main dye ingredient: chromotrope 2R. Helminth eggs generally do not stain well with any of the permanent stains, while protozoan cysts and trophozoites could be stained using the routine Wheatley's trichrome stain. Coccidian oocysts stain well using some of the modified acid-fast stains. The correct answer is D.
REFERENCES