

Chagas’ Disease (Trypanosoma cruzi, American Trypanosomiasis)
Organism:
American trypanosomiasis (Chagas’ disease) is a zoonosis caused by T. cruzi, which was discovered in the intestine of a triatomid bug in Brazil in 1909 by Carlos Chagas, who described the entire life cycle in reservoir hosts. Trypanosomes are hemoflagellates, which cause serious medical problems for humans. Trypanosomes belong to the family Trypanosomatidae that live in the blood and tissues of their human hosts.
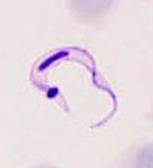
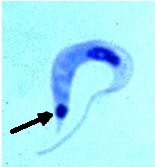
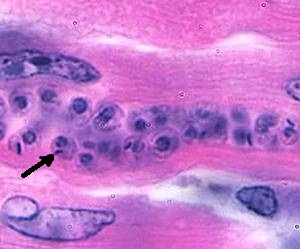
Left and Middle: Trypanosoma cruzi trypomastigote, note the arrow pointing at the very large kinetoplast; Right: Trypanosoma cruzi amastigotes in cardiac tissue (arrow pointing at individual organism).
Life Cycle:
The disease is transmitted to humans through the bite wound caused by reduviid bugs (triatomids, -kissing bugs, or conenose bugs). Humans are infected when metacyclic trypomastigotes are released with the feces while the insect is taking a blood meal and the feces are rubbed or scratched into the bite wound or onto mucosal surfaces such as eyes or mouth, an action stimulated by the allergic reaction to the insect’s saliva. The organisms can also be transmitted as congenital infections, by blood transfusion, or by organ transplantation. On entry into the body, the metacyclic forms invade local tissues, transform to the amastigote stage, and begin to multiply within the cells. The local inflammatory process continues, forming the primary lesion, the chagoma, which blocks the lymphatic capillaries and causes edema.
Epidemiology:
Chagas’ disease is a zoonosis occurring throughout American continents and involves reduviid bugs living in close association with human reservoirs (dogs, cats, armadillos, opossums, raccoons, and rodents). The most ubiquitous sylvatic reservoir host is the opossum, Didelphus, which is found throughout much of the range of T. cruzi in the Americas. Multiple nesting or resting sites of the opossum encompass many types of triatomine habitat. High T. cruzi prevalence rates are partly due to the fact that opossums eat triatomines and may also transmit infection via anal gland secretions. Sylvatic cycles of T. cruzi transmission extend from southern Argentina and Chile to northern California. Housing conditions are extremely important in transmission; the prevalence and incidence of infection are very high in human dwellings where the vector has adapted to living in the mud and in thatch walls and palm leaf roofs. The reduviid bug has easy access to humans to obtain blood meals and transmit the infection in this type of dwelling. A significant reduction in transmission was noted to occur in houses with walls made of plaster where cracks and crevices were covered, in contrast to houses with mud or thatch walls. Human infections occur mainly in rural areas where poor sanitary and socioeconomic conditions and poor housing provide excellent breeding places for reduviid bugs. These conditions allow maximum contact between the vector and humans.
Clinical Features:
In addition to contracting T. cruzi infections through the insect’s bite wound or exposed mucous membranes, persons can be infected by blood transfusion, organ transplantation, placental transfer, and accidental ingestion of parasitized reduviid bugs. The clinical syndromes associated with Chagas’ disease can be broken down into early infection, acute, indeterminate, and chronic stages. The acute stage is the result of the first encounter of the patient with the parasite, whereas the chronic phase is the result of late sequelae. In children younger than 5 years, the disease is seen in its severest form, whereas in older children and adults, the disease is milder and is commonly diagnosed in the subacute or chronic form rather than in the acute form. Overall, the incubation period in humans is about 7 to 14 days but is somewhat longer in some patients.
Early Disease. A localized inflammatory reaction of variable intensity may ensue at the infection site. In most cases, the reaction is mild and may not be apparent. An erythematous subcutaneous nodule (chagoma), seen most frequently on the face, may form. The chagoma is painful and may take 2 to 3 months to subside. In early stages of infection, amastigotes or trypomastigotes may be aspirated from the chagoma; if the route of inoculation is the ocular mucosa, edema of the eyelids and conjunctivitis may occur (Romaña’s sign). The edema does not pit on pressure, and the skin is dry. Although Romaña’s sign is thought to be almost pathognomonic for early Chagas’ disease, in an analysis of 300 patients with early disease, unilateral periorbital edema was found in only half of the patients. The infective stages spread to the regional lymph nodes, which become enlarged, hard, and tender. Similar chagoma lesions may develop in other body areas as the infection spreads hematogenously. Trypomastigotes appear in the blood about 10 days after infection and persist through the acute phase. These stages are rare or absent during the chronic phase.
Acute-Stage Disease. Acute-stage symptoms only occur in about 1% of patients, are usually seen in younger children, and are less obvious in older individuals because of the nonspecific nature of the symptoms and the lack of availability of health care. In general, symptoms last for 4 to 8 weeks and then may subside, even without therapy. Acute systemic signs occur around week 2 to 3 of infection and are characterized by high fevers, which may be intermittent, remitting, or continuous; hepatosplenomegaly; myalgia; erythematous rash; acute myocarditis; lymphadenopathy; keratitis; and subcutaneous edema of the face, legs, and feet. There may be signs of CNS involvement including meningoencephalitis, which has a very poor prognosis. Myocarditis is manifested by electrocardiographic changes, tachycardia, chest pain, and weakness.
Indeterminate Stage. Patients in the acute stage of Chagas’ disease may die within a few weeks or months, may recover, or may enter the chronic stage of infection. The patient may have a subpatent parasitemia and will develop antibodies to a variety of T. cruzi antigens. Chronic Chagas’ disease is diagnosed more commonly than the acute disease. The chronic stage may be initially asymptomatic (indeterminate stage), and even though trypomastigotes are seldom seen in peripheral blood, transmission by blood transfusion is a serious problem in areas where the disease is endemic.
Chronic Stage. Chronic Chagas’ disease demonstrates close interaction between the parasite and the host, causing a number of different clinical syndromes. Approximately 30% of patients develop chronic Chagas’ disease and experience cardiomyopathy, megacolon, and megaesophagus. Symptoms of the chronic stage are related to the damage sustained during the acute stage of the disease, the state of the host’s immune system, and the inflammatory response. Other contributing factors include the host genetic background, environmental and social factors, the genetic composition of the parasite, mixed infections, and reinfections. Chronic Chagas’ disease may develop years or decades after undetected infection or after the diagnosis of acute disease. The most frequent clinical sign of chronic Chagas’ disease in 25 to 30% of patients is cardiomyopathy manifested by cardiomegaly and conduction changes.
Laboratory Diagnosis:
Trypomastigote stages may be easily detected in the blood in young children; however, in chronic disease, this stage is rare or absent except during febrile exacerbations. Trypomastigotes may be detected in blood by using thin and thick blood films or by buffy coat concentration techniques. Although not routinely available except in specialized centers, PCR has been used to detect positive patients with as few as one trypomastigote in 20 ml of blood. Immunoassays have been used to detect antigens in the urine and sera of patients with congenital infections and those with chronic Chagas’ disease (85). Determination of antigenuria can be valuable for early diagnosis of Chagas’ disease and also for diagnosis of chronic cases in patients with conflicting serologic test results. A highly sensitive and specific chemiluminescent ELISA has been developed for blood bank screening and to monitor patients who are undergoing chemotherapy (86). Another ELISA using a recombinant antigen consisting of four different peptides has also been developed for screening blood donors and for epidemiologic studies and diagnosis.
Serologic tests used for the diagnosis of Chagas’ disease include complement fixation (Guerreiro-Machado test), indirect fluorescent-antibody tests, indirect hemagglutination tests, and ELISA. The use of synthetic peptides and recombinant antigens has improved the sensitivity and specificity of these diagnostic techniques, particularly in the diagnosis of congenital disease by using IgM and IgA antibody detection and in the evaluation of cure. Depending on the antigens used, cross-reactions have been noted to occur in patients with T. rangeli infection, leishmaniasis, syphilis, toxoplasmosis, hepatitis, leprosy, schistosomiasis, infectious mononucleosis, systemic lupus erythematosus, and rheumatoid arthritis. The Western blot method has been recommended for confirmatory serologic diagnosis of Chagas’ disease. The common occurrence of inconclusive results in blood donor screening for anti-Trypanosoma cruzi antibodies should be interpreted with caution. Blood transfusion services in Brazil and some other Latin America countries simultaneously perform two tests that use different methodologies to detect anti-T. cruzi antibodies; this is determined by legislation currently in force in Brazil. Unfortunately, total understanding of the results of low reactivity screening tests has not been achieved; thus serology screening information must be considered in association with epidemiological data.
Treatment:
Although numerous drugs have been tried, including those used to treat African trypanosomiasis and leishmaniasis, few have proven to be effective for therapy of Chagas’ disease. In acute and congenital Chagas’ disease and infections caused by laboratory accidents, treatment should be administered as soon as possible, even though in some cases symptoms are self-limited. Drug therapy has little effect on reducing the progression of chronic Chagas’ disease.
Benznidazole (RO-7-1051, Rochagan, Radanil), an imidazole derivative, is effective in reducing or suppressing parasites in the acute stages of disease but has limited capacity to produce a parasitic cure. It appears to be slightly more active and better tolerated than nifurtimox and is currently the drug of choice.
Control:
Although control of Chagas’ disease is feasible, few countries have initiated control programs because of both political and economic constraints.