

Baylisascaris procyonis (Pathogen – Tissue Nematode)
Organism:
Baylisascaris procyonis is an ascarid normally found in raccoons (Procyon lotor), has a normal ascarid‑like life cycle, causes a very serious zoonotic disease in humans, and is most often reported from North America.
 |
 |
 |
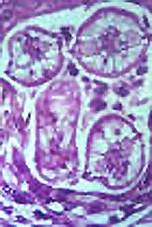 |
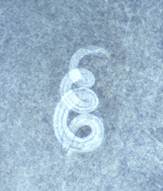
| Baylisascaris eggs | Adult worms | Larva in brain | Larvae in brain |
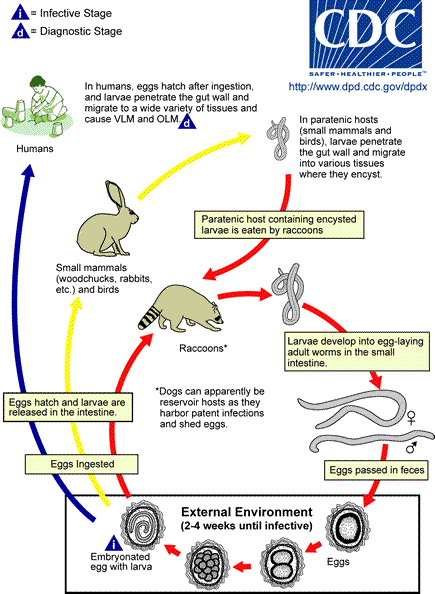

Life Cycle:
Raccoons are infected by ingesting infective eggs and by eating larvae encysted in the tissue of intermediate hosts, such as rodents, rabbits, and birds. The larvae then penetrate the mucosa of the small intestine and develop there before reentering the intestinal lumen to mature. Raccoons may be infected by up to 60 worms, and young animals have a higher prevalence of infection. These adult worms produce >150,000 eggs/worm/day; infected raccoons can shed as many as 250,000 eggs per gram of feces. This level of egg production can lead to significant environmental contamination.
Human infections result from ingestion of eggs that are passed in very large numbers (millions of eggs per day) in the feces of infected raccoons; the human then becomes the accidental intermediate host. Once ingested, the eggs hatch in the intestinal tract releasing the immature larvae. Rather than developing to adult worms as occurs in the raccoon, the larvae begin to migrate extensively throughout the body tissues, causing visceral larva migrans (VLM) and/or neural larva migrans (NLM). Although this infection presents as acute fulminant eosinophilic meningoencephalitis, two features of the life cycle are somewhat different than other helminths causing larva migrans; there is targeted migration to the central nervous system (CNS) and the continued growth of the larvae to a much larger size within the CNS. Despite different courses of therapy, there is just a single documented neurologically intact survivor of this infection. This case is the first in which spinal involvement in human Baylisascaris infection was clinically suspected and confirmed by neuroimaging. Importantly, early diagnosis and aggressive treatment of Baylisascaris meningo-encephalitis and myelitis with albendazole and high-dose steroids likely contributed to the good outcome in this patient, in contrast with previous reports (Pediatrics. 2012 Mar;129(3):e806-11. Epub 2012 Feb 6)...
Acquired:
Infection in humans is acquired through ingestion of eggs in contaminated soil.
Epidemiology:
The relationship between B. procyonis infection, raccoons, and humans has now been well defined. Recent reports of patent B. procyonis infections in dogs have caused concern because of the potential for expanded human exposure. Groups of raccoons tend to defecate in common areas called latrines, which tend to be present off the ground in fallen logs (firewood may be contaminated), rocky outcroppings, and trees. In areas that have been carefully investigated such as Pacific Grove, Calif., many latrines are present and are located directly on the ground, on roofs, in attics, and on steps and fences. These findings suggest that a very large number of raccoons are present in this location. The eggs remain viable in the soil for extended periods, often years. The eggs also have a sticky surface coating that causes them to adhere to objects, including human hands and toys. Apparently, incineration or soaking the feces with volatile solvents such as mixtures of xylene and ethanol appears to be the only means of killing the eggs (32). In some cases, removal and disposal of several inches of topsoil may also be indicated. Recognition of this new human infection and prevention of the establishment of raccoon latrine sites around areas of human habitation and recreational use are critical to successful control.
Clinical Features:
Pathologic changes due to trichinosis can be classified as (i) intestinal effects and (ii) muscle penetration and larva encapsulation. Any damage caused in either phase of the infection is usually based on the original number of ingested cysts; however, other factors such as the patient's general health, age, and size also play a role in the disease outcome. Symptoms of trichinosis are generally separated into three phases, with phase 1 being related to the presence of the parasite in the host prior to muscle invasion and phase 2 being related to the inflammatory and allergic reactions due to muscle invasion. There may also be an incubation period of up to 50 days. Phase 3 is the convalescent phase or chronic period.
Symptoms that may develop within the first 24 h include diarrhea, nausea, abdominal cramps, and general malaise, all of which may suggest food poisoning, particularly if several people are involved. Studies also indicate that the diarrhea can be prolonged, lasting up to 14 weeks (average, 5.8 weeks) with few or no muscle symptoms. It is still unknown whether this clinical presentation is related to variant biological behavior of Arctic Trichinella organisms, to previous exposure to the parasite, or to other factors.
During muscle invasion, there may be fever, facial (particularly periorbital) edema, and muscle pain, swelling, and weakness. The extraocular muscles are usually the first to be involved, followed by the muscles of the jaw and neck, limb flexors, and back. Muscle damage may cause problems in chewing, swallowing, breathing, etc., depending on which muscles are involved. The most severe symptom is myocarditis, which usually develops after the third week; death may occur between the fourth and eighth weeks. Other severe symptoms, which can occur at the same time, may involve the central nervous system (CNS). Although Trichinella encephalitis is rare, it is life threatening.
It is estimated that 10 to 20% of the patients with trichinosis have CNS involvement and that the mortality rate may reach 50% in these patients if they are not treated. Symptoms may mimic those of polyneuritis, acute anterior poliomyelitis, myasthenia gravis, meningitis, encephalitis, dermatomyositis, and polyarteritis nodosa. There may be focal paresis or paralysis (quadriplegia to single muscle group).
Peripheral eosinophilia of at least 20%, often over 50%, and possibly up to 90% is present during the muscle invasion phase of the infection. Fever can also be present at this time and can persist for several days to weeks, depending on the intensity of the infection. However, once the larvae begin to encapsulate, patient symptoms subside, and eventually the cyst wall and larvae calcify.
Clinical Specimen:
Serum: Acute and convalescent titers demonstrate several-fold increases in both serum and CSF antibody levels.
Laboratory Diagnosis:
Serology: Ocular examinations may reveal retinal lesions, larval tracks, or migrating larvae; these findings may provide the tentative diagnosis involving a helminth infection. Using IFA, ELISA and Western blot, anti-Baylisascaris antibodies can be detected in CSF. A source for serologic testing is the Department of Veterinary Pathobiology at Purdue University, West Lafayette, IN (Dr. K. Kazacos, DVM, PhD, Tel: (765) 494-7558). Acute and convalescent titers demonstrate several-fold increases in both serum and CSF antibody levels; there is no cross-reactivity with Toxocara. With the availability of a reliable serologic test, there is less need to perform a brain biopsy. In most cases, the clinical history provides the main clues.
Organism Description:
Egg: B. procyonis eggs are somewhat oval, dark brown, and measure from 63 to 88 µm by 50 to 70 µm. The eggs contain a single-celled embryo and a thick shell with a finely granular surface; they are not infective immediately after being passed, but can survive in moist soil for years. Toxocara eggs tend to be somewhat larger and have a coarsely pitted thick shell.
Larvae: Cross‑sections of larvae tend to measure 60 to 70 μm, and the larvae have prominent, single lateral alae and paired, conical excretory columns (smaller than central intestine).
Laboratory Report:
Serology results indicated (with interpretation)
Treatment:
There is no effective cure for B. procyonis infection; treatment is symptomatic and involves systemic corticosteroids and anthelmintic agents. Unfortunately, NLM is usually not responsive to anthelmintic therapy; by the time the diagnosis is made, extensive damage has already taken place. Drugs that can be tried include albendazole, mebendazole, thiabendazole, levamisole, diethylcarbamazine and ivermectin. Experimental data from animal studies indicate that albendazole and diethylcarbamazine may have the best CSF penetration and larvicidal activity. Unfortunately, since the diagnosis is considered with the beginning of symptoms, larval invasion of the CNS has already taken place. Therefore, treatment has been started late in the course of the infection.
Garcia, L.S. 2007. Diagnostic Medical Parasitology, 5th ed., ASM Press, Washington, D.C.
Control:
This infection is of great public health concern and has the potential to cause extensive damage in the human host, particularly young children. Risk is the highest for young children or infants with pica or geophagia; these individuals need to be kept away from potentially contaminated areas. Raccoons should not be encouraged to visit yards by refraining from putting out food or leaving dog food uncovered and available. Keeping pet raccoons, particularly in homes with young children, should be discouraged.