

Angiostrongyliasis, Cerebral: Angiostrongylus (Parastrongylus) cantonensis (Pathogen - Tissue Nematode)
Organism:
Human infection with the rat lungworm Angiostrongylus cantonensis was first detected in 1945 in a 15-year-old Taiwanese boy with suspected meningitis. Currently there are four species within the genus: A. cantonensis, A. costaricensis, A. malaysiensis, and A. mackerrase (these four are now placed in the new genus Parastrongylus). Since most parasitologists may continue to use the genus name Angiostrongylus, this generic designation is used.
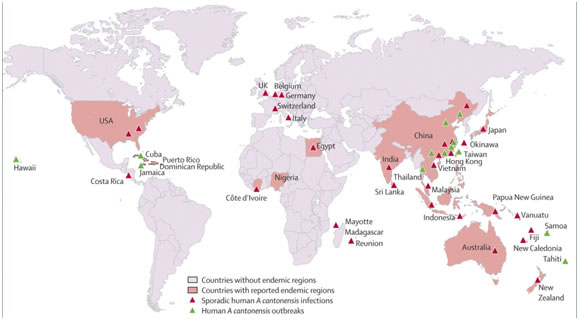
This nematode was first discovered in China in 1935, is endemic in Asia, Australia, the Caribbean islands and the Pacific Islands and has been detected on the American continent and Brazil with more than 2,800 cases of human infection reported in 30 countries. It is the cause of a global, emerging infectious disease commonly known as rat lungworm disease (RLWD). Hawaii Island is the epicenter for RLWD in the United States. According to the Hawaii Department of Health, more than 90% of all statewide cases originate from the rural east side of Hawaii Island.


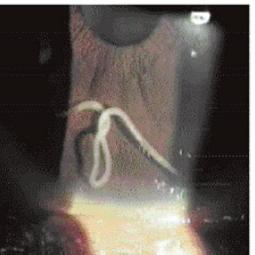
Left and Right, Angiostrongylus (Parastrongylus) cantonensis. Snail vector (common garden snail) and slug vector in Hawaii; Bottom, Male adult Angiostrongylus cantonensis in the anterior chamber of the eye.
Life Cycle:
Mature adult worms of A. cantonensis inhabit the pulmonary arteries of a wide variety of rodents, primarily those within the genera Rattus and Bandicota. Eggs laid by the female lodge in the pulmonary arteries. On hatching, the first-stage larvae enter the alveolar space, migrate up the trachea and down the alimentary tract, and are excreted in the feces. Terrestrial snails, slugs, and aquatic snails serve as intermediate hosts, either by first-stage larval penetration of tissues or by the ingestion of contaminated rodent feces. Larval development continues in the mollusk, where third-stage, infective larvae develop within about 2 weeks. When ingested by rodents, the infective third-stage larvae migrate to the brain via the circulation and develop into fourth-stage larvae and then young adults within 4 weeks. They then go to the subarachnoid space, enter the venous system, and arrive in the pulmonary arteries, where sexual maturity occurs within another 2 weeks.
Human infection begins with the accidental ingestion of infective larvae in several species of slugs, snails, or land planarians. Ingestion of infected raw paratenic hosts, including fish, amphibians, reptiles, crustaceans, and vegetables contaminated with larvae, also leads to infection of the human host. . In the natural life cycle, infective larvae have also been found in land crabs, coconut crabs, and freshwater prawns, which are often consumed raw in the Pacific Islands. In summary, human infection can originate through the following: by ingestion of L3 larvae in raw or undercooked intermediate or paratenic hosts, by drinking infected water, by oral contact with hands contaminated with mollusk larvae, or possibly through the skin.
Acquired:
Human infection begins with the accidental ingestion of infective larvae in several species of slugs, snails, or land planarians. Ingestion of infected raw paratenic hosts, including fish, amphibians, reptiles, crustaceans, and vegetables contaminated with larvae, also leads to infection of the human host. However, third-stage nematodes in paratenic hosts can cause angiostrongyliasis if the L3 are consumed by humans. The most important paratenic hosts are crustaceans (such as prawns and land crabs) and predacious land planarians (such as flatworms in the genus Platydemus). Most human infections are presumed to be due to ingestion of infected hosts (e.g. slugs, snails or other) on contaminated fresh produce.
Epidemiology:
It has been recognized in the Pacific areas for many years, with Thailand, Tahiti, and Taiwan being areas of high endemicity. Sporadic cases have also been reported in other parts of the world, including Australia, Fiji, Sri Lanka, Egypt, Madagascar, Central America, Jamaica, and Cuba. The first case in the continental United States was reported in 1995 in a patient from New Orleans. Apparently, many gastropods in New Orleans are competent hosts for A. cantonensis. Also, the presence of infected rats and primates (at the Audubon Zoo) indicates that there is a reservoir of infection in New Orleans.
Many species of snails, including the giant African snail (Achatina fulica) and apple snails (Pila and Pomacea species), and slugs act as intermediate hosts of A. cantonensis, and paratenic hosts include freshwater shrimp, frogs, and monitor lizards. Eating these hosts uncooked is a risk for developing meningitic angiostrongyliasis and the incubation period ranges from 1–90 days. Freshwater fish and shrimp, poultry, snakes, and frogs are risk factors for gnathostomiasis. Note that freshwater shrimp and frogs can carry both parasites. Unlike angiostrongyliasis, gnathostomiasis can be silent in humans for years.
Clinical Features:
The majority of angiostrongyliasis patients exhibit the meningitic form that leads to eosinophilic meningitis. The presence of an absolute count of 10 eosinophils per ml or more than 10% of the total white blood cells in the cerebrospinal fluid (CSF) meets the definition of eosinophilic meningitis and is one of the clinical clues for meningitic angiostrongyliasis. Other causes of eosinophilic meningitis are neurocysticercosis, cerebral Paragonimiasis, Toxocara canis, Baylisascaris, tuberculous meningitis, and Cryptococcal meningitis.
The incubation period is normally around 20 days but may be twice that. The main symptom in all reported cases of meningitic angiostrongyliasis was severe headache. Other symptoms include convulsions, weakness of the limbs, paresthesia, vomiting, constipation, nausea, anorexia, facial paralysis, neck stiffness, and fever. Pulmonary symptoms are usually absent, although immature adult worms have been seen in lung tissue sections. Recovery of larvae in sputum or stool has not been reported. The spinal fluid usually contains white blood cells (100 to 2,000/mm3 with many eosinophils). There is often a peripheral eosinophilia with moderate leukocytosis. In most cases, the disease is self-limiting and the patient recovers within a month. There are three major forms of human angiostrongyliasis: meningitic, encephalitic, and ocular; the majority of patients exhibit the meningitic form.
Eye involvement is characterized by visual impairment, pain, possible retinal hemorrhage, and retinal detachment. Living worms have been removed in some cases. Ocular angiostrongyliasis has been reported from Thailand, Taiwan, Vietnam, Indonesia, Japan, Papua New Guinea, and Sri Lanka. Worms have been found in the anterior chamber, retina, and other sites, causing multiple symptoms and rare cases of blindness.
Most patients, including the one from New Orleans, recover uneventfully and do not require hospitalization. Symptoms gradually disappear, with the meningeal problems resolving first, followed by the visual abnormalities and finally by the paresthesia. Although rare, the infection can be fatal.
Clinical Specimen and Organism Description:
The third-stage larvae can be identified when submitted for final review. The morphological criteria for AcL3 were a length of 422 to 525 mm, a width of 24 to 35 mm and the termination of the tail at a fine point.
Left and Right, Angiostrongylus cantonensis third stage (L3) infective larvae.
Laboratory Diagnosis:
Definitive diagnosis of infection with A. cantonensis would require identification of larvae or young adults in human tissue, such as the brain, CSF, and eye chamber, all of which can be difficult. Thus, the diagnosis is usually made on the basis of serologic test results. A presumptive diagnosis can be made in areas where infections are endemic on the basis of symptoms of severe headache, meningitis, or meningoencephalitis, with fever and ocular involvement. A peripheral eosinophilia and eosinophils in the CSF would also be highly suggestive. Lesions can also be seen in the brain by computed tomography. Larvae or young adult worms can often be recovered in the CSF, and the serologic ELISA can also provide confirmation.
Treatment:
If the worm is found in the eye, surgical removal of the worm is normally recommended. Anthelmintic agents are usually not used, although both mebendazole and thiabendazole have been tried. Mebendazole is currently the drug of choice d. However, since pathogenesis is frequently ascribed to dead or dying worms, anthelmintics should be used with caution. Corticosteroids may also eliminate some symptoms.
Control:
No overall control measures have been recommended. However, control of the spread of infected rats and mollusks to areas where A. cantonensis is not endemic will help restrict the geographic range.