

Acanthamoeba spp. (True Pathogen – Granulomatous Amebic Encephalitis/GAE) (Keratitis, Cutaneous Infections)
Organism:
The most characteristic feature of this genus is the presence of spine‑like pseudopods called acanthapodia. Several species of Acanthamoeba (A. culbertsoni, A. castellanii, A. polyphaga, A. astronyxis, A. healyi, and A. divionensis) cause granulomatous amebic encephalitis (GAE), primarily in immunosuppressed, chronically ill, or otherwise debilitated persons. These patients tend to have no relevant history involving exposure to recreational freshwater. Acanthamoeba spp. also cause amebic keratitis and corneal ulcerations, as well as cutaneous infections.
 |
 |
 |
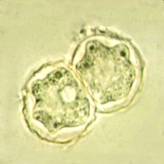
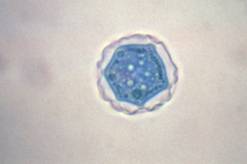
Acanthamoeba cysts |
 |
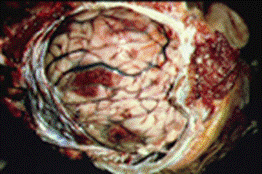 |
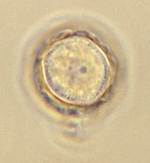
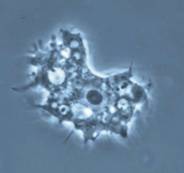
| Trophozoite wet mount | Acanthamoeba in brain |
 |
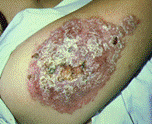 |
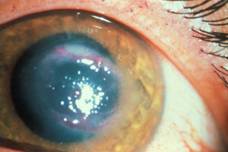
| Keratitis | Cutaneous lesion |
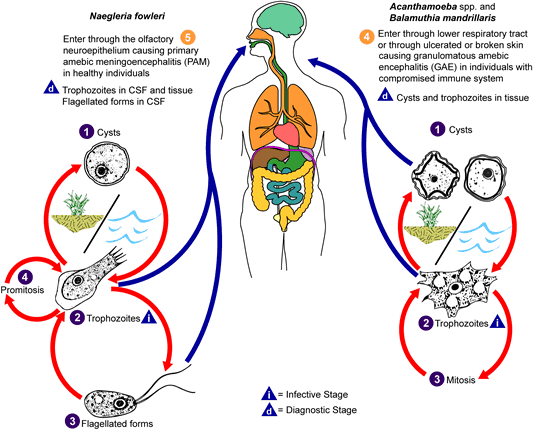
Life Cycle:
Unlike N. fowleri, Acanthamoeba spp. do not have a flagellate stage in the life cycle, only the trophozoite and cyst.
Acquired:
The amebae may enter through the lower respiratory tract or through ulcerated or broken skin, causing GAE, particularly those who are immunocompromised. With keratitis, two factors are often involved: trauma and contaminated water.
Epidemiology:
Mitochondrial DNA fingerprinting of Acanthamoeba spp. from the American Type Culture Collection and environmental sources has confirmed that approximately half of the 35 isolates displayed fingerprints similar to those of clinical isolates. Comparisons with other published mitochondrial DNA fingerprints indicated that two groups have counterparts in Europe and Japan and in Europe and Australia. These data provide strong evidence that the most common clinical isolates do have counterparts that are geographically widespread and can be isolated from the environment. Acanthamoeba cysts can remain viable in water at 4°C for 24 years. In animal studies, it has been shown that some of the isolates completely lost their virulence only after 8 years of in vitro cultivation. On the basis of these results, one can assume that cyst viability may be as long as 25 years and that these cysts can maintain their invasive properties.
Clinical Features:
GAE. Meningoencephalitis caused by Acanthamoeba spp. may present as an acute suppurative inflammation of the brain and meninges like that seen with N. fowleri infection. However, Acanthamoeba spp. are generally reported to cause a more chronic protracted slowly progressive form of meningoencephalitis (GAE). The incubation period of GAE is unknown; several weeks or months are probably necessary to establish the disease. The clinical course tends to be subacute or chronic and is usually associated with trauma or underlying disease, not swimming. GAE is characterized by confusion, dizziness, drowsiness, nausea, vomiting, headache, lethargy, stiff neck, seizures, and sometimes hemiparesis. Within the CNS, the cerebral hemispheres are the most likely tissue to be involved. There may be edema and hemorrhagic necrosis within the temporal, parietal, and occipital lobes. A chronic inflammatory exudate can be seen over the cortex, mainly of polymorphonuclear leukocytes and mononuclear cells. Unlike in PAM caused by N. fowleri, both trophozoites and cysts are found throughout the tissue. Granulomatous inflammation necrosis and thrombosed vessels are seen in the brain. Multinucleated giant cells forming granulomas can be seen in immunocompetent patients, while in immunocompromised individuals, granuloma formation is generally poor or lacking. It is unknown whether brain necrosis is caused by direct tissue destruction caused by the organisms, by inflammatory cytokines such as interleukin-1 or tumor necrosis factor, or both. Also, dissemination to other tissues such as the liver, kidneys, trachea, and adrenals can occur in immunocompromised individuals; more unusual sites also include the ear and necrotic bone from a bone graft of the mandible.
Keratitis. Keratitis, uveitis, and corneal ulceration have been associated with Acanthamoeba spp. Infections have been seen in both hard‑ and soft‑lens wearers, and particular attention has been paid to soft‑lens disinfection systems, including home‑made saline solutions. Heat disinfection overall appears to be more effective than cold disinfection methods in killing Acanthamoeba trophozoites and cysts. The onset of corneal infection with Acanthamoeba spp. can vary tremendously; however, two factors often appear to be involved: trauma and contaminated water. When corneal abrasions occur, the disease process is usually more rapid, with ulceration, corneal infiltration, iritis, scleritis, severe pain, hypopyon (pus in the anterior chamber), and loss of vision. When this process occurs in an individual who wears contact lenses, the onset is more gradual, but the results are often the same. A contact lens can act as a mechanical vector for transport of amebae present in the storage case onto the cornea. Subsequent multiplication and invasion of the tissue may occur.
Cutaneous lesions. Cutaneous infections are more common in patients with AIDS, regardless of the presence or absence of CNS involvement. The disease includes the presence of hard erythematous nodules or skin ulcers. The early lesions appear as firm papulonodules that drain purulent material; these lesions then develop into nonhealing indurated ulcers. Although disseminated skin lesions may be the first sign of Acanthamoeba infection, it is unclear whether the skin lesions represent a primary focus or may result from hematogenous spread from other body sites. Although the mortality rate for these individuals without CNS involvement is around 75%, it increases to 100% if cutaneous infection is accompanied by CNS disease
Clinical Specimen:
Tissue or Scans: In the differential diagnosis, other space‑occupying lesions of the CNS (tumor, abscess, fungal infection, etc.) must also be considered. Some of the predisposing factors have included Hodgkin's disease, diabetes, alcoholism, pregnancy, and steroid therapy. Organisms have also been found in the adrenal gland, brain, eyes, kidneys, liver, pancreas, skin, spleen, thyroid gland, and uterus.
Eye: Decreased corneal sensation has contributed to the misdiagnosis of Acanthamoeba keratitis as herpes simplex keratitis; this mistake can also be attributed to the presence of irregular epithelial lesions, stromal infiltrative keratitis, and edema, which are commonly seen in herpes simplex keratitis. Acanthamoeba keratitis may be present as a secondary or opportunistic infection in patients with herpes simplex keratitis. Unfortunately, as a result, treatment can be delayed for 2 weeks to 3 months. The presence of nonhealing corneal ulcers and the presence of ring infiltrates are also clinical signs that alert the ophthalmologist to the possibility of amebic infection.
Laboratory Diagnosis: THIS REQUEST IS ALWAYS A STAT!
Specimens should never be refrigerated prior to examination. When centrifuging the CSF, low speeds (250 ´ g) should be used so that the trophozoites are not damaged. Although bright‑field microscopy with reduced light is acceptable, phase microscopy, if available, is recommended. Use of smears stained with Giemsa or Wright's stain or a Giemsa‑Wright's stain combination can also be helpful. If N. fowleri is the causative agent, trophozoites only are normally seen. If the infecting organism is Acanthamoeba spp., cysts may also be seen in specimens from CNS infection. Unfortunately, most cases are diagnosed at autopsy; confirmation of these tissue findings must include culture and/or special staining with monoclonal reagents in indirect fluorescent antibody procedures. Organisms can also be cultured on nonnutrient agar plated with Escherichia coli.
The most effective approach uses nonnutrient agar plates with Page's saline and an overlay growth of Escherichia coli on which the amebae feed. Tissue stains are also effective, and cysts can be stained with Gomori's silver methenamine, periodic acid‑Schiff, and calcofluor white.
The amebae can be identified in histologic preparations by indirect immunofluorescence and immunoperoxidase techniques. The organism in tissue sections looks very much like an Iodamoeba bütschlii trophozoite, with a very large karyosome and no peripheral nuclear chromatin; the organisms can also be seen with routine histologic stains.
Organism Description:
Trophozoite Motile organisms have spine-like pseudopods; however, progressive movement is usually not very evident. There is a wide range (25 to 40 µm), with the average diameter of the trophozoites being 30 μm. The nucleus has the typical large karyosome, like that seen in N. fowleri. This morphology can be seen on a wet preparation.
Cyst: The cysts are usually round with a single nucleus, also having the large karyosome seen in the trophozoite nucleus. The double wall is usually visible, with the slightly wrinkled outer cyst wall and what has been described as a polyhedral inner cyst wall. This cyst morphology can be seen in organisms cultured on agar plates. Cyst formation occurs under adverse environmental condition; they are resistant to biocides, chlorination, and antibiotics. The cysts can also survive low temperatures (0 to 2ºC).
Culture: The most effective approach uses nonnutrient agar plates with Page's saline and an overlay growth of Escherichia coli on which the amebae feed. There is also evidence that phosphate‑buffered saline can be used. Specimens transported in ameba‑saline (5.0 ml) and filtered through 13‑mm, 0.22‑μm‑pore‑size cellulose acetate and nitrate filters (Millipore Corp., Bedford, Mass.) have also been acceptable for organism recovery. The filter is then placed in the center of the nonnutrient agar plate seeded with E. coli. Tissue stains are also effective, and cysts can be stained with Gomori's silver methenamine, periodic acid‑Schiff, and calcofluor white. Both cysts and trophozoites can be recovered from culture.
Laboratory Report:
Acanthamoeba spp.seen and/or cultured
Treatment:
Trophozoites and cysts of Acanthamoeba isolates vary in their sensitivity to antimicrobial agents. They are sensitive in vitro to ketoconazole, pentamidine, hydroxystilbamidine, paromomycin, 5-fluorocytosine, polymyxin, sulfadiazine, trimethoprim-sulfamethoxazole, azithromycin, and extracts of medicinal plants and, especially, to combinations of these drugs. Increased awareness of these infections and early therapy play a large role in patient outcomes. The use of drug combinations helps address resistance that may exist or occur during treatment. In vitro testing confirms strain and species differences in sensitivity, so that no single drug is effective against all amebae.
Because amebic keratitis may be misdiagnosed and/or therapy delayed, total blindness may result in the infected eye(s). However, if the infection is correctly diagnosed early and the epithelium alone is involved, debridement may be sufficient for cure. This approach not only removes organisms, but enhances the delivery of topical medications. However, if treatment is delayed and the organisms invade the cornea or tissues below the cornea, therapy may need to be continued for many months to a year or longer. These patients must continually be monitored because the cysts tend to be quite resistant to therapy; treatment failures tend to be fairly common. In vitro susceptibility testing may be very helpful in these cases
Garcia, L.S. 2007. Diagnostic Medical Parasitology, 5th ed., ASM Press, Washington, D.C.
Control:
General preventive measures are similar to those for N. fowleri. Several disinfectants have been tested against both Naegleria spp. and Acanthamoeba spp. The conclusion is that Naegleria spp. are generally susceptible to swimming pool levels of chlorine but Acanthamoeba spp. are more resistant. The recovery of Acanthamoeba spp. in nasal isolates and pharyngeal swabs may indicate human introduction of the organisms into swimming pools. With the increasing reports of infection in wearers of soft contact lenses, it will be important to carefully consider the effectiveness of various contact lens disinfection systems. A number of factors probably play a role in the increased incidence of infection: a large number of HIV-infected individuals and more patients undergoing cancer chemotherapy or immunosuppressive therapy for organ transplantation.