Table 3.6 Diagnostic Medical Parasitology
Calibration of Microscope with an Ocular Micrometer
PREANALYTICAL CONSIDERATIONS
I. PRINCIPLE
he identification of protozoa and other parasites depends on several factors, one of which is size. Any laboratory doing diagnostic work in parasitology should have a calibrated microscope available for precise measurements. Measurements are made with a micrometer disk that is placed in the ocular of the microscope; the disk is usually calibrated as a line divided into 50 units (U). Depending on the objective magnification used, the divisions in the disk represent different measurements. The ocular disk division must be compared with a known calibrated scale, usually a stage micrometer with a scale of 0.1- and 0.01-mm divisions (1) (Fig. 9.3.2–1).
II. MATERIALS
- A. Supplies
- 1. Ocular micrometer disk (line divided into 50 U) (any laboratory supply distributor: Fisher, Baxter, Scientific Products, VWR, etc.)
- 2. Stage micrometer with a scale of 0.1- and 0.01-mm divisions (Fisher, Baxter, Scientific Products, VWR, etc.)
- 3. Immersion oil
- 4. Lens paper
- B. Equipment
- 1. Binocular microscope with 10×, 40×, and 100× objectives. Other objective magnifications (50× oil or 60× oil immersion lenses) may also be used.
- 2. Oculars should be 10×. Some may prefer 5×; however, smaller magnification may make final identifications more difficult.
- 3. Single 10× ocular to be used to calibrate all laboratory microscopes (to be used when any organism is being measured)
ANALYTICAL CONSIDERATIONS
III. QUALITY CONTROL
- A. Recalibrate the microscope periodically. If the scope receives heavy use, once a year is recommended, or when any parts are replaced or changed on the microscope. If the microscope is roughly bumped and/or knocked over, it must be recalibrated prior to use. In these cases, professional service and/or repair may be required prior to recalibration.
- B. The measurement of RBCs (approximately 7.5 μm) is recommended to check the calibrations of the three magnifications (×100, ×400, ×1,000).
- C. Latex or polystyrene beads of a standardized diameter can be used to check the calculations and measurements; however, they tend to be quite expensive (Applied Physics, Inc, www.appliedphysicsusa.com ; ALPHA Nanotech, www.alphananotechne.com ) and often the shelf life is approximately 1 y). Most products are primarily used for fluorescence microscopy. (Sigmaaldrich, www.sigmaaldrich.com ; Thermofisher, www.thermofisher.com ).
- D. Record all measurements in QC records.
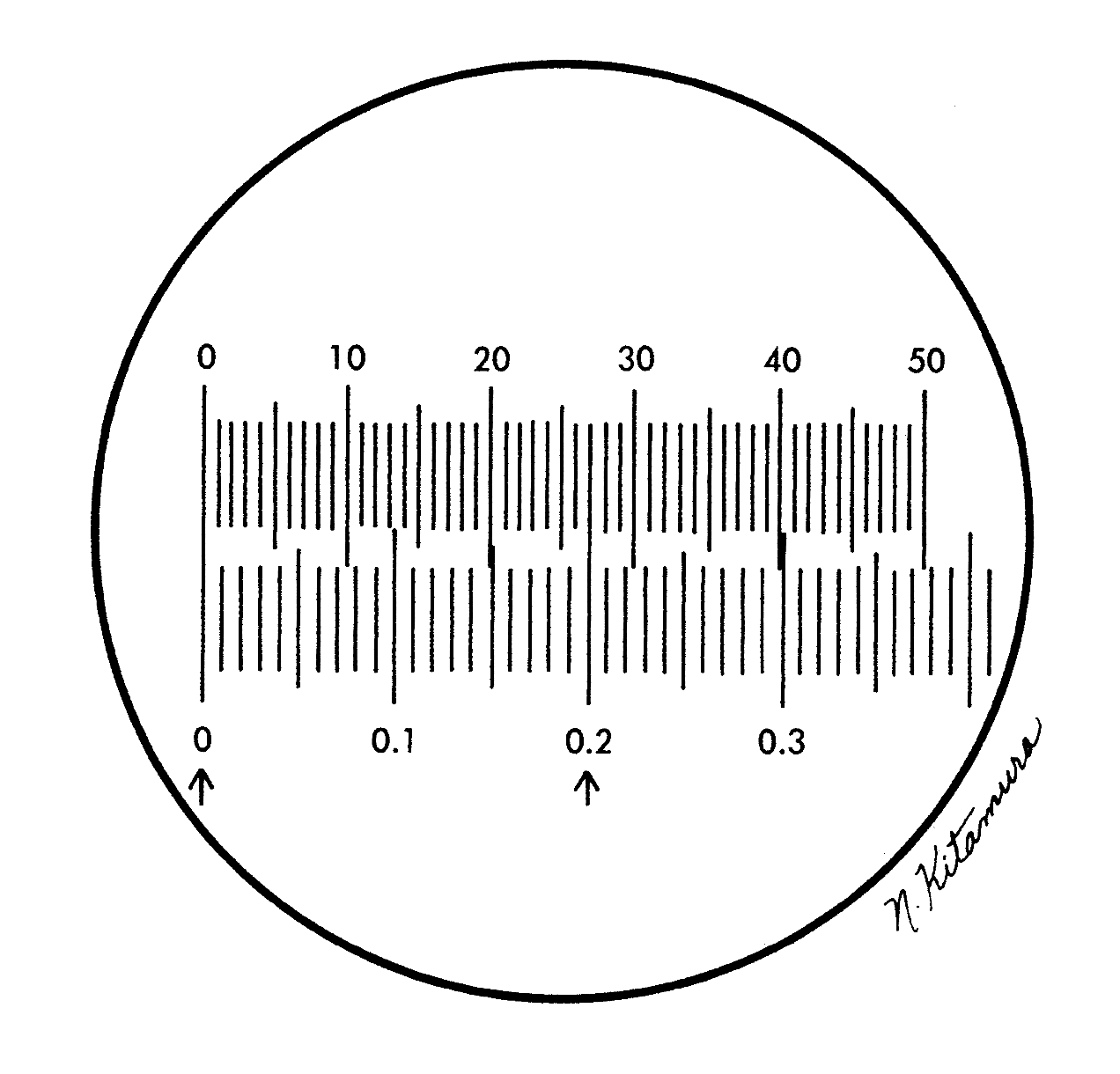
Figure 9.3.2–1 Ocular micrometer, top scale; stage micrometer, bottom scale (from Garcia LS, Diagnostic Medical Parasitology, 6th ed, 2016, ASM Press, Washington, DC).
IV. PROCEDURE
- A. Unscrew the eye lens of a 10× ocular, and place the micrometer disk (engraved side down) within the ocular. Use two thicknesses of lens paper to handle the disk; keep all surfaces free of dust or lint. A single thickness of lens paper will not protect the disk and/or lenses from oil transfer on your fingers.
- B. Place the calibrated micrometer on the stage, and focus on the scale. You should be able to distinguish the difference between the 0.1- and 0.01-mm divisions. Make sure you understand the divisions on the scale before proceeding.
- C. Adjust the stage micrometer so that the “0” line on the ocular micrometer is exactly lined up on top of the 0 line on the stage micrometer.
- D. When these two 0 lines are lined up, do not move the stage micrometer any farther. Look to the right of the 0 lines for another set of lines superimposed on each other. The second set of lines should be as far to the right of the 0 lines as possible; however, the distance varies with the objectives being used (Fig. 9.3.2–1).
- E. Count the number of ocular divisions between the 0 lines and the point where the second set of lines is superimposed. Then, on the stage micrometer, count the number of 0.1-mm divisions between the 0 lines and the second set of superimposed lines.
- F. Calculate the portion of a millimeter that is measured by a single small ocular unit.
- G. When the high dry and oil immersion objectives are used, the 0 line of the stage micrometer will increase in size, whereas the ocular 0 line will remain the same size. The thin ocular 0 line should be lined up in the center or at one edge of the broad stage micrometer 0 line. Thus, when the second set of superimposed lines is found, the thin ocular line should be lined up in the center or at the corresponding edge of the broad stage micrometer line.
- H. Calculate the factors as follows. In addition to the measurement, it is important to consider potential organism morphology as well. There may be one or more organisms that measure within the same size range.
- Examples:
- Stage reading (mm)/ocular reading × 1,000 μm/1 mm = ocular units (μm)
- Low power (10×): (0.8 mm/100 U) × (1,000 μm/1 mm) = 8.0 μm (factor)
- High dry power (40×): (0.1 mm/50 U) × (1,000 μm/1 mm) = 2.0 μm (factor)
- Oil immersion (100×): (0.05 mm/62 U) × (1,000 μm/1 mm) = 0.8 μm (factor)
- Examples:
- If a helminth egg measures 12 ocular units by 7 ocular units with the low power objective, then multiply the measurements by the factor 8.0 μm (for that objective). The egg then measures 96 by 56 μm and falls within the size range of Paragonimus spp. trematode eggs.
- If a helminth egg measures 15 ocular units by 7 ocular units with the high dry objective, then multiply the measurements by the factor 2.0 μm (for that objective). The egg then measures 30 by 14 μm and is probably Clonorchis sinensis or one of the smaller trematode eggs.
- If a protozoan cyst measures 27 ocular units with the oil immersion objective, then multiply the measurement by the factor 0.8 μm (for that objective). The cyst then measures 21.6 μm. Identification would depend on visible morphological characteristics, since more than one protozoan cyst could measure 21.6 μm.
- If a protozoan cyst measures 10 ocular units with the oil immersion objective (permanent stained smear), then multiply the measurement by the factor 0.8 (for that objective). The cyst then measures 8 microns and is most probably Entamoeba hartmanni; this assumes the morphology is consistent with the Entamoeba spp. complex. This measurement would include any clear shrinkage halo around the cyst due to permanent staining reagents.
V. RESULTS
- A. For each objective magnification, a factor will be generated (1 ocular unit = certain number of micrometers).
- B. If standardized latex or polystyrene beads or an RBC is measured with various objectives, the size for the object measured should be the same (or very close), regardless of the objective magnification.
POSTANALYTICAL CONSIDERATIONS
VI. REPORTING RESULTS
- A. Post the multiplication factor for each objective either on the base of the microscope or on a nearby wall or bulletin board for easy reference. Remember that each microscope with the three or more objectives calibrated together cannot be substituted for other equipment from another microscope. If objective/scope substitutions are made, the entire system (microscope and objectives used) must be recalibrated prior to use for accurate measurements. Although the objectives will be marked the same (10x, 40x, 100x oil immersion), the actual calibration figures will vary among different microscopes.
- B. Once the number of ocular lines per width and length of the organism is measured, then, depending on the objective magnification, the factor (1 ocular unit = certain number of micrometers) can be applied to the number of lines to obtain the width and length of the organism.
- C. Comparison of these measurements with reference measurements in various books and manuals should confirm the organism identification.
VII. PROCEDURE NOTES
- A. The final multiplication factors will be only as good as your visual comparison of the ocular 0 and stage micrometer 0 lines.
- B. The high dry objective (40×) factor should be approximately 2.5 times more than the factor obtained from the oil immersion objective (100×). The low-power objective (10×) factor should be approximately 10 times that of the oil immersion objective (100×).
VIII. LIMITATIONS OF THE PROCEDURE
- A. After each objective has been calibrated, the oculars containing the disk and/or these objectives cannot be interchanged with corresponding objectives or oculars on another microscope.
- B. Each microscope used to measure organisms must be calibrated as a unit. The original oculars and objectives that were used to calibrate the microscope must also be used when an organism is measured.
- C. The objective containing the ocular micrometer can be stored until needed. This single ocular can be inserted when measurements are taken. However, this particular ocular containing the ocular micrometer disk must also have been used as the ocular during microscope calibration.
REFERENCE
1. Garcia LS. 2016. Diagnostic Medical Parasitology, 6th ed. ASM Press, Washington, DC.


