What we have
Featured Products
MCC provides a wide range of medical chemical solutions to meet your specific application.
The original Pre-Activated and Ready to Use 2.65% glutaraldehyde solution we formulated over 40 years ago.
Total-Fix is a patented single vial stool fixative: No mercury, No formalin, No PVA. Compatible with permanent staining, fecal concentration, and lateral flow, micro-well EIA and FA assays for Giardia and Cryptosporidium. Also for molecular platforms.
Cera-Pel paraffin repellent allows for quick and easy cleanup of paraffin on countertops and around equipment.
Product scent: Mild Cucumber
WHAt we offer
Featured Product Lines
A chemical material or compound made use of to suspend or destroy microbes on inert surfaces.
Transport vials, Fixatives, Stains and FA Kit to identify parasites and parasitic eggs in fecal samples.
Various stains and reagents used to help with the diagnosis of infections and diseases.
Stains and reagents used to transport, fix, process and stain tissues for disease and cancer diagnosis.
work process
We Complete Every Step Carefully
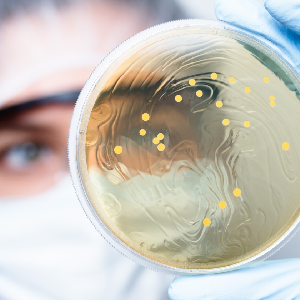
Bacti-Stain
MCC provides a convenient method of confirming and differentiating morphology of positive slides with the traditional stains.
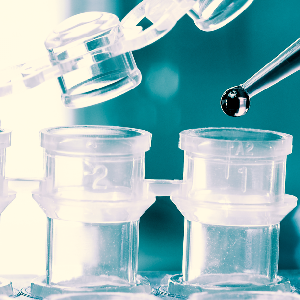
Concentration Systems
3 different systems (2 patented) for fecal concentration. Two closed and one open system utilizing 3ml to 30ml of fecal specimen to detect parasites and parasitic eggs.
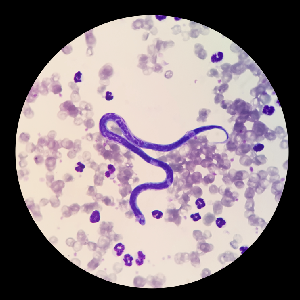
Parasitology
Procedures and products to aid the technologist in the identification of parasites and parasite eggs.

60+
Years of experience

Quick
Turnaround Time

Best
Quality Guarantee

4
Patented Products

We Provide OEM Services
We are the leading source of your private label manufacturer








