

Filariasis, Loa loa (Pathogen – Tissue Nematode)
Organism:
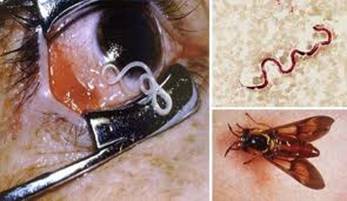
Loa loa, the African eye worm, was first noted in the eye of a Negro girl in the West Indies in 1770. In 1895, Argyll‑Robertson described the adult worms that he extracted from the eye of a woman who had resided at Old Calabar in West Africa. The adult worms migrate through the subcutaneous tissues, producing intermittent "Calabar swellings," in addition to migrating beneath the conjunctiva. Approximately 13 million people are infected with L. loa in Central and West Africa. The vectors are biting flies (Chrysops) (mango or deerflies) and are known as red flies in Africa.
 |
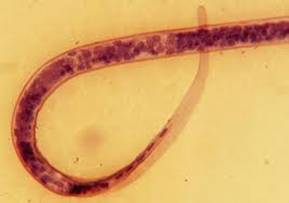 |
| Loa loa | Loa loa tail nuclei |
 |
 |
 |
| Loa loa in eye | Calabar swellings |
Life Cycle:
The adult male and female worms live and migrate in the subcutaneous and deep connective tissues, and the microfilariae are found in the blood, where they can be ingested by mango flies or deerflies (Chrysops spp.). Once ingested by the fly, the microfilariae of L. loa lose their sheaths, penetrate the gut wall, migrate to the fat body, undergo two molts, and become infective in approximately 10 to 12 days. Humans are infected when bitten by infected flies, and the infective larvae enter the skin through the bite wound. Development into adult worms takes about 6 to 12 months and they can survive up to 17 years.
Acquired:
Infection in humans is acquired through the bite of infected Chrysops flies.
Epidemiology:
Loiasis is endemic to the Central and West African rain forests and may infect as many as 13 million people. Disease distribution depends on the vectors, which breed in wet mud at the side of streams under the rain forest canopy. Flies appear to be attracted by the movement of people or vehicles, as well as rising smoke. An excellent example of vector‑host interactive sites would include rubber plantations with a dense high canopy and human workers. The Chrysops flies are more common in the rainy season, and exposure differences result in higher infection rates in adults rather than children.
Clinical Features:
The Chrysops bite results in erythema, swelling, and itching, symptoms which can worsen with the presence of infective larvae. The adult worms normally migrate through the subcutaneous tissue at a rate of about 1 cm/min. This migration is not painful and is seldom noticed unless the worm is passing over the bridge of the nose or through the conjunctiva of the eye. Many patients with active L. loa infections do not have a microfilaremia.
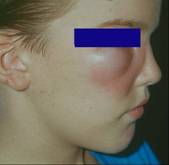
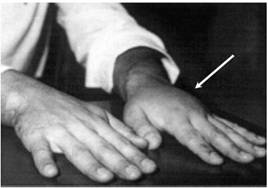
The most common pathologic sequelae associated with L. loa infections are Calabar or fugitive swellings (angioedema). Calabar swellings are localized subcutaneous edemas, a type of inflammatory reaction brought about by a host response to the worm or its metabolic products. Calabar swellings may be found anywhere on the body but predominate on the extremities. The swellings develop rapidly over a few hours and may be preceded by localized pain, pruritus, and urticaria; the swellings usually last 1 to 3 days. Serious complications due to loiasis have included cardiomyopathy, encephalopathy, nephropathy, and pleural effusions.
Clinical Specimen:
Blood: In areas of endemic infection around the world, the presumptive diagnosis of filarial infections is frequently based on clinical evidence; however, definitive diagnosis is based on the detection of microfilariae, primarily in the blood.
Laboratory Diagnosis:
Blood: The diagnostic methods are similar to those for W. bancrofti and include thin and thick blood films, wet preparations and concentrations, PCR, ultrasonography, antigen, and antibody detection. Since the microfilariae of L. loa do not appear in the blood until years after the adult worms or the host reaction to infection is noted, the diagnosis is frequently made on the basis of clinical history (Calabar swellings, worm migrations through the eye, eosinophilia, and residence in an area of endemicity). Microfilariae can be detected in the blood by using methods similar to those used to detect W. bancrofti or B. malayi. Microfilariae are shed by the females on a random schedule; therefore, multiple samples over a period of days may have to be tested. Individuals not from the area of endemicity usually are amicrofilaremic (63). The microfilariae exhibit diurnal periodicity; therefore, blood samples should be collected during daytime, preferably between 10 a.m. and 3 p.m. Since Giemsa stain will not reveal the sheath, Delafield’s hematoxylin is recommended.
Organism Description:
Adult: The females measure 50 to 70 by 0.5 mm, while the males measure 30 to 35 by 0.3 to 0.4 mm.
Microfilariae: The microfilariae frequently are not detected in the blood until years after the adult worms are noted. The microfilariae have a diurnal periodicity whose peak occurs about midday; the remainder of the time they can be found in pulmonary capillaries. The microfilariae are sheathed and are 250 to 300 μm long. When stained, the body nuclei are continuous to the tip of the tail.
Laboratory Report:
Microfilariae identified and reported
Treatment:
Surgical removal of the adult worms as they are migrating across the bridge of the nose or through the conjunctiva is relatively simple. Diethylcarbamazine (DEC) is an effective treatment; however, in patients with a heavy microfilaremia, anti‑inflammatory drugs may also have to be administered to reduce severe side effects. The most serious sequelae are neurologic complications resembling the encephalitis that is commonly associated with a high microfilaremia. If neurological disorders develop, it is mandatory that therapy with DEC be stopped. Some individuals may require multiple courses of therapy to be clinically cured. Although DEC is curative in most individuals, relapses can occur within the first 12 months and up to 8 years after treatment.
Both mebendazole and ivermectin have been used to treat loiasis. The use of mebendazole is not promising, whereas ivermectin is effective in reducing microfilaremia. Albendazole, a benzimidazole derivative related to mebendazole, is effective in reducing microfilaremia, and there is a lower risk of encephalopathy; however, repeated courses of therapy may be required. Individuals treated with albendazole have a significant reduction in the levels of eosinophils, antifilarial IgG, and IgG4. Although there were no cures, there is the suggestion that albendazole directly affects the adult stages.
Garcia, L.S. 2007. Diagnostic Medical Parasitology, 5th ed., ASM Press, Washington, D.C.
Control:
Although vector control is one means of prevention, the inaccessibility of breeding sites has proven to be a problem. Clearing of growth around houses and use of screens and protective clothing have all been helpful. In general, control of loiasis also depends to a great degree on chemoprophylaxis with DEC. Although other therapeutic approaches have been tried, including mebendazole and ivermectin, problems remain with both options.